Page 1215 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1215
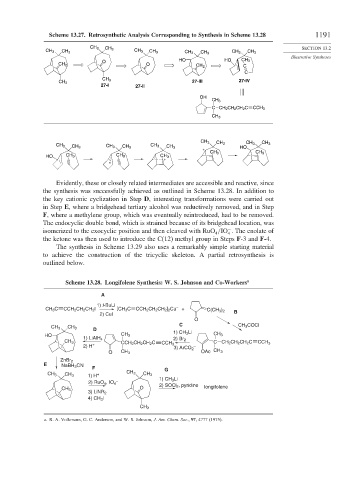
Scheme 13.27. Retrosynthetic Analysis Corresponding to Synthesis in Scheme 13.28 1191
CH 3 CH 3 SECTION 13.2
CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 CH 3
Illustrative Syntheses
HO HO
O CH 3
CH 2 O CH 2 C
C
CH 3 27-III 27-IV
CH 3
27-I 27-II
OH
CH 3
C CH 2 CH 2 CH 2 C CCH 3
CH 3
CH 3
CH 3 CH 3 CH 3
CH 3 CH 3 CH 3 CH 3 CH 3 CH 3 HO
+
CH 3 CH 3
HO CH 3 CH 3 CH 3
+
+
Evidently, these or closely related intermediates are accessible and reactive, since
the synthesis was successfully achieved as outlined in Scheme 13.28. In addition to
the key cationic cyclization in Step D, interesting transformations were carried out
in Step E, where a bridgehead tertiary alcohol was reductively removed, and in Step
F, where a methylene group, which was eventually reintroduced, had to be removed.
The endocyclic double bond, which is strained because of its bridgehead location, was
−
isomerized to the exocyclic position and then cleaved with RuO /IO . The enolate of
4 4
the ketone was then used to introduce the C(12) methyl group in Steps F-3 and F-4.
The synthesis in Scheme 13.29 also uses a remarkably simple starting material
to achieve the construction of the tricyclic skeleton. A partial retrosynthesis is
outlined below.
Scheme 13.28. Longifolene Synthesis: W. S. Johnson and Co-Workers a
A
1) t-BuLi
–
CH 3 C CCH 2 CH 2 CH 2 I [CH 3 C CCH 2 CH 2 CH 2 ] 2 Cu + C(CH 3 ) 2 B
2) CuI
O
C CH 3 COCl
CH 3 CH 3
D
HO CH 3 1) CH 3 Li CH 3
1) LiAlH 4 2) Br 2
CH 3 CCH 2 CH 2 CH 2 C CCH 3 C CH 2 CH 2 CH 2 C CCH 3
2) H + –
3) ArCO 2
O CH 3 OAc CH 3
ZnBr 2
E NaBH 3 CN
F
G
CH 3 CH 3 1) H + CH 3 CH 3
– 1) CH 3 Li
2) RuO 4 , IO 4 2) SOCl 2 , pyridine
O longifolene
CH 3
3) LiNR 2
4) CH 3 I
CH 3
a. R. A. Volkmann, G. C. Anderson, and W. S. Johnson, J. Am. Chem. Soc., 97, 4777 (1975).