Page 1220 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1220
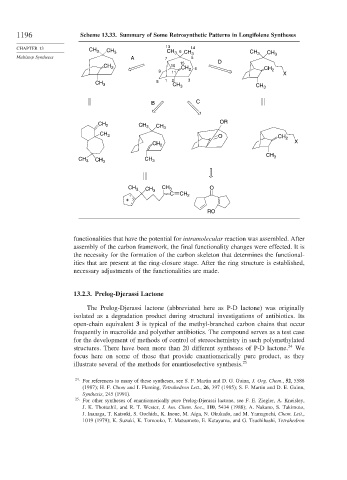
1196 Scheme 13.33. Summary of Some Retrosynthetic Patterns in Longifolene Syntheses
CHAPTER 13 CH 3 CH 3 13 6 CH 14 CH
CH 3
Multistep Syntheses A 7 5 3 CH 3 3
15 D
CH 2 10 CH CH
9 11 2 4 2 X
CH 3 8 1 2 CH 3 3 CH 3
B C
OR
CH 2 CH 3 CH 3
CH 3 O CH 2
CH 2 X
CH
CH 3 CH 3 CH 3 3
CH 3 CH CH 3 O
3
C CH 2
+
RO
functionalities that have the potential for intramolecular reaction was assembled. After
assembly of the carbon framework, the final functionality changes were effected. It is
the necessity for the formation of the carbon skeleton that determines the functional-
ities that are present at the ring-closure stage. After the ring structure is established,
necessary adjustments of the functionalities are made.
13.2.3. Prelog-Djerassi Lactone
The Prelog-Djerassi lactone (abbreviated here as P-D lactone) was originally
isolated as a degradation product during structural investigations of antibiotics. Its
open-chain equivalent 3 is typical of the methyl-branched carbon chains that occur
frequently in macrolide and polyether antibiotics. The compound serves as a test case
for the development of methods of control of stereochemistry in such polymethylated
structures. There have been more than 20 different syntheses of P-D lactone. 24 We
focus here on some of those that provide enantiomerically pure product, as they
illustrate several of the methods for enantioselective synthesis. 25
24 For references to many of these syntheses, see S. F. Martin and D. G. Guinn, J. Org. Chem., 52, 5588
(1987); H. F. Chow and I. Fleming, Tetrahedron Lett., 26, 397 (1985); S. F. Martin and D. E. Guinn,
Synthesis, 245 (1991).
25
For other syntheses of enantiomerically pure Prelog-Djerassi lactone, see F. E. Ziegler, A. Kneisley,
J. K. Thottathil, and R. T. Wester, J. Am. Chem. Soc., 110, 5434 (1988); A. Nakano, S. Takimoto,
J. Inanaga, T. Katsuki, S. Ouchida, K. Inoue, M. Aiga, N. Okukado, and M. Yamaguchi, Chem. Lett.,
1019 (1979); K. Suzuki, K. Tomooko, T. Matsumoto, E. Katayama, and G. Tsuchihashi, Tetrahedron