Page 1206 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1206
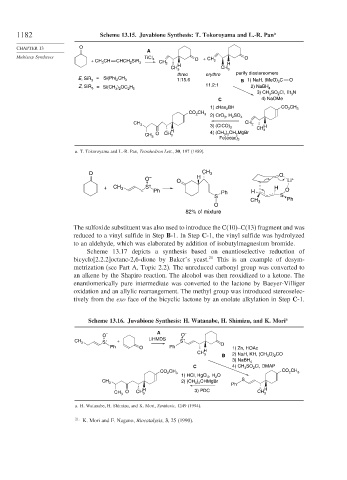
1182 Scheme 13.15. Juvabione Synthesis: T. Tokoroyama and L.-R. Pan a
CHAPTER 13 O
A
Multistep Syntheses + CH O
+ CH CH CHCH SiR TiCl 4 CH O 2
3 2 3 2 H H
CH 3 CH 3
threo erythro purify diastereomers
E, SiR 3 = Si(Ph) CH 3 1:15.6 B 1) NaH, (MeO) C O
2
2
Z, SiR 3 = Si(CH ) OC H 5 11.2:1 2) NaBH 4
3 2
2
3) CH SO Cl, Et N
3
3
2
C 4) NaOMe
1) cHex BH CO CH
2 2 3
CO CH 3 2) CrO , H SO
2
3 2 4
CH 3 3) (ClCO) CH 2 H
2
H 4) (CH ) CH MgBr CH 3
CH 3 O CH 3 3 2 2
Fe(acac) 2
a. T. Tokoroyama and L.-R. Pan, Tetrahedron Lett., 30, 197 (1989).
O CH 3 O
O – O H Li +
+ CH 3 S + H
– Ph Ph H O
S S
CH 3 Ph
O
82% of mixture
The sulfoxide substituent was also used to introduce the C(10)–C(13) fragment and was
reduced to a vinyl sulfide in Step B-1. In Step C-1, the vinyl sulfide was hydrolyzed
to an aldehyde, which was elaborated by addition of isobutylmagnesium bromide.
Scheme 13.17 depicts a synthesis based on enantioselective reduction of
bicyclo[2.2.2]octane-2,6-dione by Baker’s yeast. 21 This is an example of desym-
metrization (see Part A, Topic 2.2). The unreduced carbonyl group was converted to
an alkene by the Shapiro reaction. The alcohol was then reoxidized to a ketone. The
enantiomerically pure intermediate was converted to the lactone by Baeyer-Villiger
oxidation and an allylic rearrangement. The methyl group was introduced stereoselec-
tively from the exo face of the bicyclic lactone by an enolate alkylation in Step C-1.
Scheme 13.16. Juvabione Synthesis: H. Watanabe, H. Shimizu, and K. Mori a
A
O – O –
LiHMDS +
CH 3 S + + S
Ph O Ph O 1) Zn, HOAc
CH H O) CO
3 2) NaH, KH, (CH 3
B 2
3) NaBH 4
C 4) CH SO Cl, DMAP
3
2
CO CH CO CH 3
2
2 3 , H O
1) HCl, HgCl 2
2
CH 2) (CH 3 2 S
) CHMgBr
3
Ph
H 3) PDC H
O CH CH 3
CH 3 3
a. H. Watanabe, H. Shimizu, and K. Mori, Synthesis, 1249 (1994).
21
K. Mori and F. Nagano, Biocatalysis, 3, 25 (1990).