Page 1237 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1237
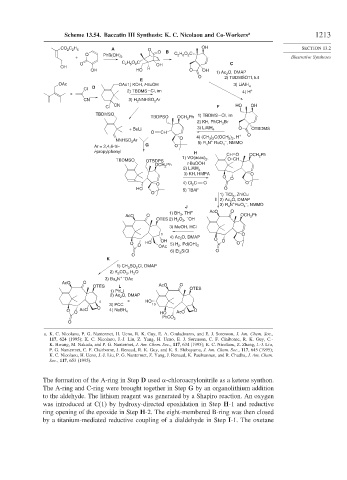
Scheme 13.54. Baccatin III Synthesis: K. C. Nicolaou and Co-Workers a 1213
C H OH SECTION 13.2
CO 2 2 5 A O B
H O C
O PhB(OH) O C 2 5
+ 2 2 Illustrative Syntheses
H O C
O C 2 5 2 OH C
OH H
OH HO O OH 1) Ac O, DMAP
2
O 2) TBDMSOTf, lut
E
OAc OAc1) KOH, t-BuOH 3) LiAlH 4
Cl D 2) TBDMS Cl, im +
+ 4) H
CN 3) H NNHSO Ar
2
2
CN HO OH
Cl F
TBDMSO 1) TBDMS Cl, im
TBDPSO OCH 2 Ph
2) KH, PhCH Br
2
+ BuLi 3) LiAlH 4 O OTBDMS
O CH O
O 4) (CH ) C(OCH ) , H +
3 2
3 2
NNHSO Ar
+
–
2 5) R N RuO , NMMO
Ar = 2,4,6-tri- G O 4 4
i-propylphenyl H CH O Ph
1) VO(acac) , OCH 2
TBDMSO OTBDPS 2 O CH
Ph t-BuOOH
OCH 2
2) LiAlH 4
3) KH, HMPA O
O
O
O 4) Cl C O O
2
HO 5) TBAF O
O , Zn/Cu
1) TiCl 3
O, DMAP
I 2) Ac 2
+
–
N RuO , NMMO
J 3) R 4 4
1) BH 3 , THF AcO O
AcO O – OCH 2 Ph
OTES 2) H 2 O , OH
2
3) MeOH, HCl
5 O
4) Ac 2 O, DMAP O
O HO OH 5) H , Pd(OH) O O
O OAc 2 2
6) Et SiCl O
O 3
K
1) CH SO Cl, DMAP
3 2
CO , H O
2) K 2 3 2
+ –
3) Bu N OAc
AcO O 4
OTES L AcO O OTES
1) PhLi
2) Ac O, DMAP
2
5 HO
3) PCC 13
O AcO O 4) NaBH 4 O
O HO AcO
PhCO 2
O
a. K. C. Nicolaou, P. G. Nantermet, H. Ueno, R. K. Guy, E. A. Couladouros, and E. J. Sorenson, J. Am. Chem. Soc.,
117, 624 (1995); K. C. Nicolaou, J.-J. Liu, Z. Yang, H. Ueno, E. J. Sorenson, C. F. Claiborne, R. K. Guy, C.-
K. Hwang, M. Nakada, and P. G. Nantermet, J. Am. Chem. Soc., 117, 634 (1995); K. C. Nicolaou, Z. Zhang, J.-J. Liu,
P. G. Nantermet, C. F. Clairborne, J. Renaud, R. K. Guy, and K. S. Shibayama, J. Am. Chem. Soc., 117, 645 (1995);
K. C. Nicolaou, H. Ueno, J.-J. Liu, P. G. Nantermet, Z. Yang, J. Renaud, K. Paulvannan, and R. Chadha, J. Am. Chem.
Soc., 117, 653 (1995).
The formation of the A-ring in Step D used -chloroacrylonitrile as a ketene synthon.
The A-ring and C-ring were brought together in Step G by an organolithium addition
to the aldehyde. The lithium reagent was generated by a Shapiro reaction. An oxygen
was introduced at C(1) by hydroxy-directed epoxidation in Step H-1 and reductive
ring opening of the epoxide in Step H-2. The eight-membered B-ring was then closed
by a titanium-mediated reductive coupling of a dialdehyde in Step I-1. The oxetane