Page 1239 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1239
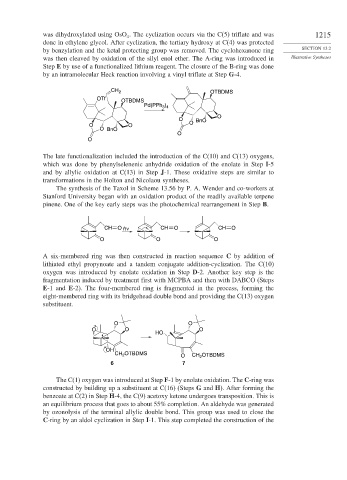
was dihydroxylated using OsO . The cyclization occurs via the C(5) triflate and was 1215
4
done in ethylene glycol. After cyclization, the tertiary hydroxy at C(4) was protected
by benzylation and the ketal protecting group was removed. The cyclohexanone ring SECTION 13.2
was then cleaved by oxidation of the silyl enol ether. The A-ring was introduced in Illustrative Syntheses
Step E by use of a functionalized lithium reagent. The closure of the B-ring was done
by an intramolecular Heck reaction involving a vinyl triflate at Step G-4.
CH 2 OTBDMS
OTf OTBDMS
Pd(PPh )
3 4
O
O BnO
O O O
O BnO
O
O
The late functionalization included the introduction of the C(10) and C(13) oxygens,
which was done by phenylselenenic anhydride oxidation of the enolate in Step I-5
and by allylic oxidation at C(13) in Step J-1. These oxidative steps are similar to
transformations in the Holton and Nicolaou syntheses.
The synthesis of the Taxol in Scheme 13.56 by P. A. Wender and co-workers at
Stanford University began with an oxidation product of the readily available terpene
pinene. One of the key early steps was the photochemical rearrangement in Step B.
CH O h ν CH O CH O
O O O
A six-membered ring was then constructed in reaction sequence C by addition of
lithiated ethyl propynoate and a tandem conjugate addition-cyclization. The C(10)
oxygen was introduced by enolate oxidation in Step D-2. Another key step is the
fragmentation induced by treatment first with MCPBA and then with DABCO (Steps
E-1 and E-2). The four-membered ring is fragmented in the process, forming the
eight-membered ring with its bridgehead double bond and providing the C(13) oxygen
substituent.
O O
O O O
HO
OH
CH OTBDMS O CH OTBDMS
2
2
6 7
The C(1) oxygen was introduced at Step F-1 by enolate oxidation. The C-ring was
constructed by building up a substituent at C(16) (Steps G and H). After forming the
benzoate at C(2) in Step H-4, the C(9) acetoxy ketone undergoes transposition. This is
an equilibrium process that goes to about 55% completion. An aldehyde was generated
by ozonolysis of the terminal allylic double bond. This group was used to close the
C-ring by an aldol cyclization in Step I-1. This step completed the construction of the