Page 1236 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1236
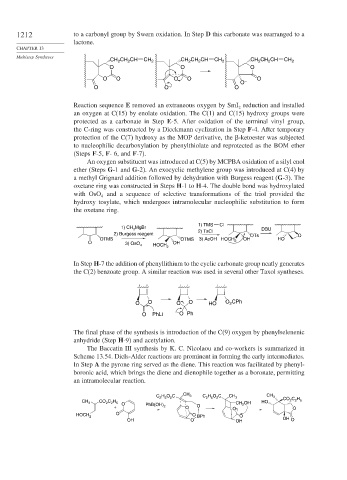
1212 to a carbonyl group by Swern oxidation. In Step D this carbonate was rearranged to a
lactone.
CHAPTER 13
Multistep Syntheses
2
2
CH 2 CH CH CH 2 CH 2 CH 2 CH CH 2 CH CH CH CH 2
2
O O O
O O O O O
O –
O O – O
Reaction sequence E removed an extraneous oxygen by SmI reduction and installed
2
an oxygen at C(15) by enolate oxidation. The C(1) and C(15) hydroxy groups were
protected as a carbonate in Step E-5. After oxidation of the terminal vinyl group,
the C-ring was constructed by a Dieckmann cyclization in Step F-4. After temporary
protection of the C(7) hydroxy as the MOP derivative, the -ketoester was subjected
to nucleophilic decarboxylation by phenylthiolate and reprotected as the BOM ether
(Steps F-5, F-6,and F-7).
An oxygen substituent was introduced at C(5) by MCPBA oxidation of a silyl enol
ether (Steps G-1 and G-2). An exocyclic methylene group was introduced at C(4) by
a methyl Grignard addition followed by dehydration with Burgess reagent (G-3). The
oxetane ring was constructed in Steps H-1 to H-4. The double bond was hydroxylated
with OsO and a sequence of selective transformations of the triol provided the
4
hydroxy tosylate, which undergoes intramolecular nucleophilic substitution to form
the oxetane ring.
1) TMS Cl
1) CH MgBr
3 2) TsCl DBU
2) Burgess reagent OTs O
OTMS OTMS 3) AcOH HOCH OH HO
O OH 2
HOCH 2
3) OsO 4
In Step H-7 the addition of phenyllithium to the cyclic carbonate group neatly generates
the C(2) benzoate group. A similar reaction was used in several other Taxol syntheses.
O O O O HO O CPh
2
O PhLi – O Ph
The final phase of the synthesis is introduction of the C(9) oxygen by phenylselenenic
anhydride (Step H-9) and acetylation.
The Baccatin III synthesis by K. C. Nicolaou and co-workers is summarized in
Scheme 13.54. Diels-Alder reactions are prominent in forming the early intermediates.
In Step A the pyrone ring served as the diene. This reaction was facilitated by phenyl-
boronic acid, which brings the diene and dienophile together as a boronate, permitting
an intramolecular reaction.
C H O C CH 3 C H O C CH 3 CH 3
2 5
2
2 5
2
2 2 5
CH 3 CO C H O OH HO CO C H
2 2 5
+ PhB(OH) 2 O O O CH 2 O
HOCH O O BPh O
2 OH
OH O OH O