Page 1245 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1245
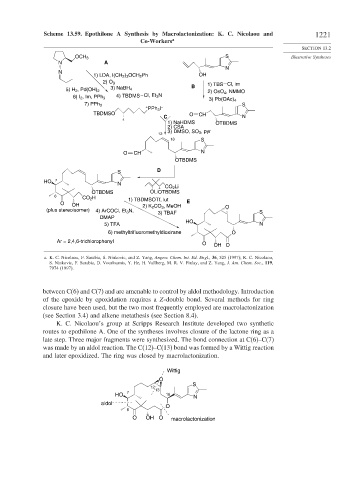
Scheme 13.59. Epothilone A Synthesis by Macrolactonization: K. C. Nicolaou and 1221
Co-Workers a
SECTION 13.2
S
OCH 3 Illustrative Syntheses
N A
N
N
1) LDA, I(CH 2 ) 3 OCH 2 Ph OH
2) O 3 1) TBS Cl, im
B
3) NaBH 4
5) H 2 , Pd(OH) 2 2) OsO 4 , NMMO
4) TBDMS Cl, Et 3 N
6) I 2 , Im, PPh 3
3) Pb(OAc) 4
7) PPh 3 S
+ –
PPh 3 I
TBDMSO O CH
C N
1) NaHDMS OTBDMS
2) CSA
3) DMSO, SO 3 , pyr
12
13 S
O CH N
OTBDMS
S D
HO 7
N
CO 2 Li
OTBDMS OLiOTBDMS
6
CO 2 H 1) TBDMSOTf, lut
O OH 2) K 2 CO 3 , MeOH E
(plus stereoisomer) 4) ArCOCl, Et 3 N, 3) TBAF O S
DMAP
5) TFA HO N
6) methyltrifluoromethyldioxirane O
Ar = 2,4,6-trichlorophenyl
O OH O
a. K. C. Nicolaou, F. Sarabia, S. Ninkovic, and Z. Yang, Angew. Chem. Int. Ed. Engl., 36, 525 (1997); K. C. Nicolaou,
S. Ninkovic, F. Sarabia, D. Vourloumis, Y. He, H. Vallberg, M. R. V. Finlay, and Z. Yang, J. Am. Chem. Soc., 119,
7974 (1997).
between C(6) and C(7) and are amenable to control by aldol methodology. Introduction
of the epoxide by epoxidation requires a Z-double bond. Several methods for ring
closure have been used, but the two most frequently employed are macrolactonization
(see Section 3.4) and alkene metathesis (see Section 8.4).
K. C. Nicolaou’s group at Scripps Research Institute developed two synthetic
routes to epothilone A. One of the syntheses involves closure of the lactone ring as a
late step. Three major fragments were synthesized. The bond connection at C(6)–C(7)
was made by an aldol reaction. The C(12)–C(13) bond was formed by a Wittig reaction
and later epoxidized. The ring was closed by macrolactonization.
Wittig
O
S
12
7 13
HO 16 N
aldol O
6
O OH O macrolactonization