Page 1267 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1267
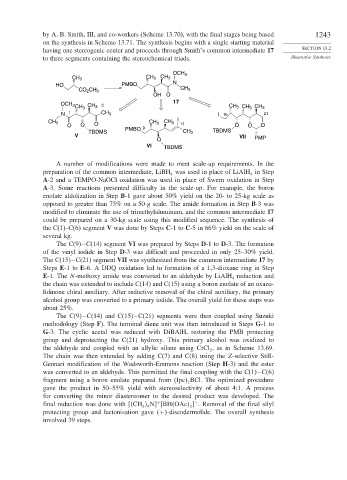
by A. B. Smith, III, and co-workers (Scheme 13.70), with the final stages being based 1243
on the synthesis in Scheme 13.71. The synthesis begins with a single starting material
having one stereogenic center and proceeds through Smith’s common intermediate 17 SECTION 13.2
to three segments containing the stereochemical triads. Illustrative Syntheses
OCH 3
CH 3 CH 3 CH 3
HO PMBO N
CH 3
CO 2 CH 3
OH O
17
OCH 3 CH 3 6
CH 3 CH 3 CH 3 CH 3
N 1 CH 3 I 15 21
I
CH 3 O CH 3 CH 3 14
O O O O O
PMBO 9
TBDMS CH 3 TBDMS
V VII PMP
O
VI TBDMS
A number of modifications were made to meet scale-up requirements. In the
preparation of the common intermediate, LiBH was used in place of LiAlH in Step
4
4
A-2 and a TEMPO-NaOCl oxidation was used in place of Swern oxidation in Step
A-3. Some reactions presented difficulty in the scale-up. For example, the boron
enolate aldolization in Step B-1 gave about 50% yield on the 20- to 25-kg scale as
opposed to greater than 75% on a 50-g scale. The amide formation in Step B-3 was
modified to eliminate the use of trimethylaluminum, and the common intermediate 17
could be prepared on a 30-kg scale using this modified sequence. The synthesis of
the C(1)–C(6) segment V was done by Steps C-1 to C-5 in 66% yield on the scale of
several kg.
The C(9)−C(14) segment VI was prepared by Steps D-1 to D-3. The formation
of the vinyl iodide in Step D-3 was difficult and proceeded in only 25–30% yield.
The C(15)−C(21) segment VII was synthesized from the common intermediate 17 by
Steps E-1 to E-6. A DDQ oxidation led to formation of a 1,3-dioxane ring in Step
E-1. The N-methoxy amide was converted to an aldehyde by LiAlH reduction and
4
the chain was extended to include C(14) and C(15) using a boron enolate of an oxazo-
lidinone chiral auxiliary. After reductive removal of the chiral auxiliary, the primary
alcohol group was converted to a primary iodide. The overall yield for these steps was
about 25%.
The C(9)−C(14) and C(15)−C(21) segments were then coupled using Suzuki
methodology (Step F). The terminal diene unit was then introduced in Steps G-1 to
G-3. The cyclic acetal was reduced with DiBAlH, restoring the PMB protecting
group and deprotecting the C(21) hydroxy. This primary alcohol was oxidized to
the aldehyde and coupled with an allylic silane using CrCl , as in Scheme 13.69.
2
The chain was then extended by adding C(7) and C(8) using the Z-selective Still-
Gennari modification of the Wadsworth-Emmons reaction (Step H-3) and the ester
was converted to an aldehyde. This permitted the final coupling with the C(1)−C(6)
fragment using a boron enolate prepared from Ipc BCl. The optimized procedure
2
gave the product in 50–55% yield with stereoselectivity of about 4:1. A process
for converting the minor diastereomer to the desired product was developed. The
−
final reduction was done with
CH N
BH OAc . Removal of the final silyl
+
3 4
3
protecting group and lactonization gave + -discodermolide. The overall synthesis
involved 39 steps.