Page 1281 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1281
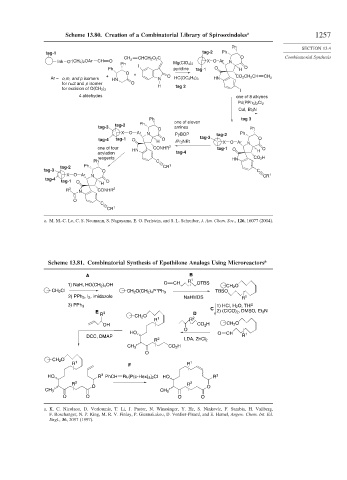
Scheme 13.80. Creation of a Combinatorial Library of Spirooxindoles a 1257
Ph SECTION 13.4
tag-1 tag-2 Ph
CH CHCH 2 O 2 C O Combinatorial Synthesis
link O – (CH 2 ) 2 OAr CH O 2 X O Ar N
Ph I Mg(ClO 4 ) 2
Ph pyridine tag-1 O H O
O
+ + O CO 2 CH 2 CH CH
Ar = o,m, and p isomers HN N HC(OC 2 H 5 ) 3 HN 2
for n=2 and p isomer O
H tag 2
for excision of O(CH 2 ) 2
I
4 aldehydes one of 8 alkynes
Pd(PPh 3 ) 2 Cl 2
CuI, Et 3 N
Ph tag 3
tag-2 Ph one of eleven
tag-3 amines
O Ph
X O Ar N PyBOP tag-2 Ph
tag-4 tag-1 O H O iPr 2 NEt tag-3 X O Ar N O
one of four HN CONHR 2 tag-1 O O
acylation tag-4 H
reagents HN CO 2 H
Ph C
tag-2 Ph CR 1
tag-3 O C
X O Ar N CR 1
tag-4
tag-1 O H O
R 3 N CONHR 2
O
C
CR 1
a. M. M.-C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein, and S. L. Schreiber, J. Am. Chem. Soc., 126, 16077 (2004).
Scheme 13.81. Combinatorial Synthesis of Epothilone Analogs Using Microreactors a
A B
R 1
1) NaH, HO(CH 2 ) 4 OH O CH OTBS CH 2 O
+
CH 2 Cl CH 2 O(CH 2 ) 4 P Ph 3 TBSO
2) PPh 3 , I 2 , imidazole NaHMDS R 1
3) PPh 3 1) HCl, H 2 O, THF
C
E 3 D 2) (ClCO) 2 , DMSO, Et 3 N
R CH 2 O
R 1 R 2
OH CO 2 H CH 2 O
O
HO O CH
DCC, DMAP R 1
R 2 LDA, ZnCl 2
CO 2 H
CH 3
O
CH 2 O
R 1 F R 1
3
HO R PhCH Ru[P(c-Hex) 3 ] 2 Cl HO R 3
R 2 R 2
O O
CH 3 CH 3
O O O O
a. K. C. Nicolaou, D. Vorloumis, T. Li, J. Pastor, N. Winssinger, Y. He, S. Ninkovic, F. Sarabia, H. Vallberg,
F. Roschanger, N. P. King, M. R. V. Finlay, P. Giannakakou, D. Verdier-Pinard, and E. Hamel, Angew. Chem. Int. Ed.
Engl., 36, 2097 (1997).