Page 1280 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1280
1256 I
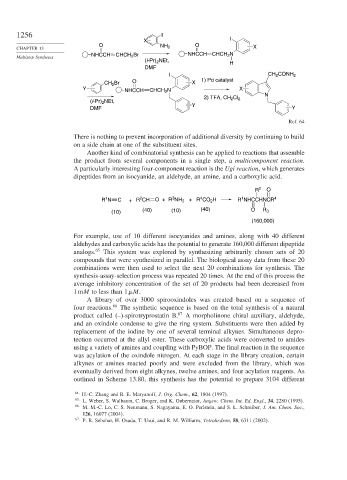
X I
O O
CHAPTER 13 NH 2 X
NHCCH CHCH 2 Br NHCCH CHCH 2 N
Multistep Syntheses
(i-Pr) 2 NEt,
H
DMF
I CH 2 CONH 2
CH 2 Br O X 1) Pd catalyst
Y NHCCH CHCH 2 N X
N
2) TFA, CH 2 Cl 2
(i-Pr) 2 NEt,
Y
DMF Y
Ref. 64
There is nothing to prevent incorporation of additional diversity by continuing to build
on a side chain at one of the substituent sites.
Another kind of combinatorial synthesis can be applied to reactions that assemble
the product from several components in a single step, a multicomponent reaction.
A particularly interesting four-component reaction is the Ugi reaction, which generates
dipeptides from an isocyanide, an aldehyde, an amine, and a carboxylic acid.
R 2 O
3
2
1
4
1
R N C + R CH O + R NH 2 + R CO 2 H R NHCCHNCR 4
(10) (40) (10) (40) O R 3
(160,000)
For example, use of 10 different isocyanides and amines, along with 40 different
aldehydes and carboxylic acids has the potential to generate 160,000 different dipeptide
analogs. 65 This system was explored by synthesizing arbitrarily chosen sets of 20
compounds that were synthesized in parallel. The biological assay data from these 20
combinations were then used to select the next 20 combinations for synthesis. The
synthesis-assay-selection process was repeated 20 times. At the end of this process the
average inhibitory concentration of the set of 20 products had been decreased from
1mM to less than 1 M.
A library of over 3000 spirooxindoles was created based on a sequence of
four reactions. 66 The synthetic sequence is based on the total synthesis of a natural
product called (–)-spirotryprostatin B. 67 A morpholinone chiral auxiliary, aldehyde,
and an oxindole condense to give the ring system. Substituents were then added by
replacement of the iodine by one of several terminal alkynes. Simultaneous depro-
tection occurred at the allyl ester. These carboxylic acids were converted to amides
using a variety of amines and coupling with PyBOP. The final reaction in the sequence
was acylation of the oxindole nitrogen. At each stage in the library creation, certain
alkynes or amines reacted poorly and were excluded from the library, which was
eventually derived from eight alkynes, twelve amines, and four acylation reagents. As
outlined in Scheme 13.80, this synthesis has the potential to prepare 3104 different
64
H.-C. Zhang and B. E. Maryanoff, J. Org. Chem., 62, 1804 (1997).
65 L. Weber, S. Walbaum, C. Broger, and K. Gubernator, Angew. Chem. Int. Ed. Engl., 34, 2280 (1995).
66 M. M.-C. Lo, C. S. Neumann, S. Nagayama, E. O. Perlstein, and S. L. Schreiber, J. Am. Chem. Soc.,
126, 16077 (2004).
67
P. R. Sebahar, H. Osada, T. Usui, and R. M. Williams, Tetrahedron, 58, 6311 (2002).