Page 1275 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1275
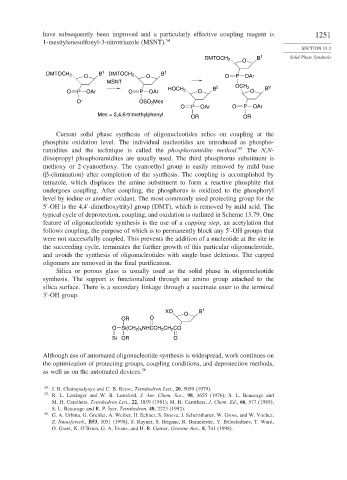
have subsequently been improved and a particularly effective coupling reagent is 1251
1-mesitylenesulfonyl-3-nitrotriazole (MSNT). 54
SECTION 13.3
DMTOCH 2 B 1 Solid Phase Synthesis
O
DMTOCH 2 B 1 DMTOCH 2 B 1
O O O P OAr
MSNT
O P OAr O P OAr HOCH 2 O B 2 OCH 2 O B 2
O – OSO 2 Mes
O P OAr O P OAr
Mes = 2,4,6-trimethylphenyl
OR OR
Current solid phase synthesis of oligonucleotides relies on coupling at the
phosphite oxidation level. The individual nucleotides are introduced as phospho-
ramidites and the technique is called the phosphoramidite method. 55 The N,N-
diisopropyl phosphoramidites are usually used. The third phosphorus substituent is
methoxy or 2-cyanoethoxy. The cyanoethyl group is easily removed by mild base
( -elimination) after completion of the synthesis. The coupling is accomplished by
tetrazole, which displaces the amine substituent to form a reactive phosphite that
undergoes coupling. After coupling, the phosphorus is oxidized to the phosphoryl
level by iodine or another oxidant. The most commonly used protecting group for the
5 -OH is the 4,4 -dimethoxytrityl group (DMT), which is removed by mild acid. The
typical cycle of deprotection, coupling, and oxidation is outlined in Scheme 13.79. One
feature of oligonucleotide synthesis is the use of a capping step, an acetylation that
follows coupling, the purpose of which is to permanently block any 5 -OH groups that
were not successfully coupled. This prevents the addition of a nucleotide at the site in
the succeeding cycle, terminates the further growth of this particular oligonucleotide,
and avoids the synthesis of oligonucleotides with single-base deletions. The capped
oligomers are removed in the final purification.
Silica or porous glass is usually used as the solid phase in oligonucleotide
synthesis. The support is functionalized through an amino group attached to the
silica surface. There is a secondary linkage through a succinate ester to the terminal
3 -OH group.
XO O B 1
OR O
O Si(CH 2 ) 5 NHCCH 2 CH 2 CO
Si OR O
Although use of automated oligonucleotide synthesis is widespread, work continues on
the optimization of protecting groups, coupling conditions, and deprotection methods,
as well as on the automated devices. 56
54 J. B. Chattapadyaya and C. B. Reese, Tetrahedron Lett., 20, 5059 (1979).
55 R. L. Letsinger and W. B. Lunsford, J. Am. Chem. Soc., 98, 3655 (1976); S. L. Beaucage and
M. H. Caruthers, Tetrahedron Lett., 22, 1859 (1981); M. H. Caruthers, J. Chem. Ed., 66, 577 (1989);
S. L. Beaucage and R. P. Iyer, Tetrahedron, 48, 2223 (1992).
56
G. A. Urbina, G. Grubler, A. Weiber, H. Echner, S. Stoeva, J. Schernthaner, W. Gross, and W. Voelter,
Z. Naturforsch., B53, 1051 (1998); S. Rayner, S. Brignac, R. Bumeiester, Y. Belosludtsev, T. Ward,
O. Grant, K. O’Brien, G. A. Evans, and H. R. Garner, Genome Res., 8, 741 (1998).