Page 1273 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1273
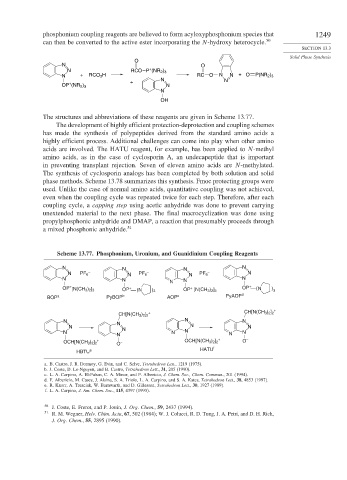
phosphonium coupling reagents are believed to form acyloxyphosphonium species that 1249
can then be converted to the active ester incorporating the N-hydroxy heterocycle. 50
SECTION 13.3
Solid Phase Synthesis
O
N O
+
N RCO P (NR 2 ) 3
N + RCO 2 H RC O N N + O P(NR 2 ) 3
N N
+
+
OP (NR 2 ) 3 N
N
OH
The structures and abbreviations of these reagents are given in Scheme 13.77.
The development of highly efficient protection-deprotection and coupling schemes
has made the synthesis of polypeptides derived from the standard amino acids a
highly efficient process. Additional challenges can come into play when other amino
acids are involved. The HATU reagent, for example, has been applied to N-methyl
amino acids, as in the case of cyclosporin A, an undecapeptide that is important
in preventing transplant rejection. Seven of eleven amino acids are N-methylated.
The synthesis of cyclosporin analogs has been completed by both solution and solid
phase methods. Scheme 13.78 summarizes this synthesis. Fmoc protecting groups were
used. Unlike the case of normal amino acids, quantitative coupling was not achieved,
even when the coupling cycle was repeated twice for each step. Therefore, after each
coupling cycle, a capping step using acetic anhydride was done to prevent carrying
unextended material to the next phase. The final macrocyclization was done using
propylphosphonic anhydride and DMAP, a reaction that presumably proceeds through
a mixed phosphonic anhydride. 51
Scheme 13.77. Phosphonium, Uronium, and Guanidinium Coupling Reagents
N N N N
N PF 6 – N PF 6 – N PF 6 – N
N N N N
N
+ + OP +
+
OP [N(CH 3 ) 2 ] 3 OP (N )3 OP [N(CH 3 ) 2 ] 3 (N ) 3
BOP a PyBOP b AOP c PyAOP d
+
+ CH[N(CH 3 ) 2 ] 2
CH[N(CH 3 ) 2 ] 2
N N N N
N N N N
N N N N N
N
+ + O –
OCH[N(CH 3 ) 2 ] 2 – OCH[N(CH 3 ) 2 ] 2
O
HBTU e HATU f
a. B. Castro, J. R. Dormoy, G. Evin, and C. Selve, Tetrahedron Lett., 1219 (1975).
b. J. Coste, D. Le-Nguyen, and B. Castro, Tetrahedron Lett., 31, 205 (1990).
c. L. A. Carpino, A. El-Faban, C. A. Minor, and F. Albericio, J. Chem. Soc., Chem. Commun., 201 (1994).
d. F. Albericio, M. Cases, J. Alsina, S. A. Triolo, L. A. Carpino, and S. A. Kates, Tetrahedron Lett., 38, 4853 (1997).
e. R. Knorr, A. Trezciak, W. Barnwarth, and D. Gillessen, Tetrahedron Lett., 30, 1927 (1989).
f. L. A. Carpino, J. Am. Chem. Soc., 115, 4397 (1993).
50 J. Coste, E. Frerot, and P. Jouin, J. Org. Chem., 59, 2437 (1994).
51
R. M. Wegner, Helv. Chim. Acta, 67, 502 (1984); W. J. Colucci, R. D. Tung, J. A. Petri, and D. H. Rich,
J. Org. Chem., 55, 2895 (1990).