Page 17 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 17
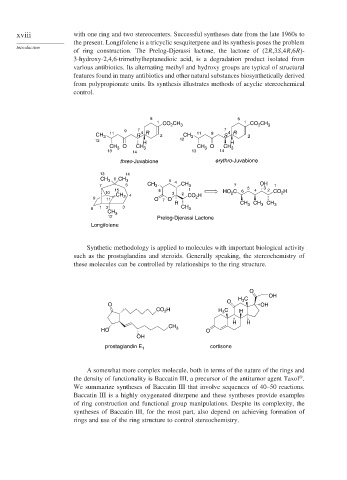
xviii with one ring and two stereocenters. Successful syntheses date from the late 1960s to
the present. Longifolene is a tricyclic sesquiterpene and its synthesis poses the problem
Introduction
of ring construction. The Prelog-Djerassi lactone, the lactone of (2R,3S,4R,6R)-
3-hydroxy-2,4,6-trimethylheptanedioic acid, is a degradation product isolated from
various antibiotics. Its alternating methyl and hydroxy groups are typical of structural
features found in many antibiotics and other natural substances biosynthetically derived
from polypropionate units. Its synthesis illustrates methods of acyclic stereochemical
control.
6 6
1 CH 1 CO CH
CO 2 3 2 3
9 7 7 4
CH 3 11 R 4 R 2 CH 3 11 9 S R 2
12 H 12 H
CH 3 O CH 3 CH 3 O CH 3
13 14 13 14
threo-Juvabione erythro-Juvabione
13 14
CH 3 6 CH 3 5
7 5 CH 3 4 CH 3 7 OH 1
15 6 1 5 4 3 2 H
10 CH 3 2 HO 2 C 6 CO 2
2
9 11 2 4 O 7 O CO H
H CH 3 CH 3 CH 3
8 1 2 3 CH 3
CH 3
12 Prelog-Djerassi Lactone
Longifolene
Synthetic methodology is applied to molecules with important biological activity
such as the prostaglandins and steroids. Generally speaking, the stereochemistry of
these molecules can be controlled by relationships to the ring structure.
O
OH
O H 3 C
O OH
CO H H C H
2
3
H H
CH 3
HO O
OH
prostaglandin E 1 cortisone
A somewhat more complex molecule, both in terms of the nature of the rings and
®
the density of functionality is Baccatin III, a precursor of the antitumor agent Taxol .
We summarize syntheses of Baccatin III that involve sequences of 40–50 reactions.
Baccatin III is a highly oxygenated diterpene and these syntheses provide examples
of ring construction and functional group manipulations. Despite its complexity, the
syntheses of Baccatin III, for the most part, also depend on achieving formation of
rings and use of the ring structure to control stereochemistry.