Page 13 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 13
xiv
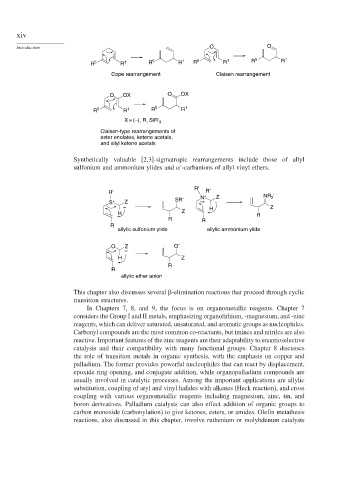
Introduction O O
R 5 R 1 R 5 R 1 R 5 R 1 R 5 R 1
Cope rearrangement Claisen rearrangement
O OX O OX
R 5 R 1 R 5 R 1
X = (–), R, SiR' 3
Claisen-type rearrangements of
ester enolates, ketene acetals,
and silyl ketene acetals
Synthetically valuable [2,3]-sigmatropic rearrangements include those of allyl
sulfonium and ammonium ylides and -carbanions of allyl vinyl ethers.
R'
R' R'
N + Z NR '
2
SR'
S + Z –
– H Z
H Z R
R R
R
allylic sulfonium ylide allylic ammonium ylide
O Z O –
–
H Z
R
R
allylic ether anion
This chapter also discusses several -elimination reactions that proceed through cyclic
transition structures.
In Chapters 7, 8, and 9, the focus is on organometallic reagents. Chapter 7
considers the Group I and II metals, emphasizing organolithium, -magnesium, and -zinc
reagents, which can deliver saturated, unsaturated, and aromatic groups as nucleophiles.
Carbonyl compounds are the most common co-reactants, but imines and nitriles are also
reactive. Important features of the zinc reagents are their adaptability to enantioselective
catalysis and their compatibility with many functional groups. Chapter 8 discusses
the role of transition metals in organic synthesis, with the emphasis on copper and
palladium. The former provides powerful nucleophiles that can react by displacement,
epoxide ring opening, and conjugate addition, while organopalladium compounds are
usually involved in catalytic processes. Among the important applications are allylic
substitution, coupling of aryl and vinyl halides with alkenes (Heck reaction), and cross
coupling with various organometallic reagents including magnesium, zinc, tin, and
boron derivatives. Palladium catalysts can also effect addition of organic groups to
carbon monoxide (carbonylation) to give ketones, esters, or amides. Olefin metathesis
reactions, also discussed in this chapter, involve ruthenium or molybdenum catalysts