Page 15 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 15
xvi Radical reactions used in synthesis include additions to double bonds, ring closure, and
atom transfer reactions. Several sequences of tandem reactions have been developed
Introduction
that can close a series of rings, followed by introduction of a substituent. Allylic
stannanes are prominent in reactions of this type.
Chapter 11 reviews aromatic substitution reactions including electrophilic
aromatic substitution, substitution via diazonium ions, and metal-catalyzed nucleophilic
substitution. The scope of the latter reactions has been greatly expanded in recent years
by the development of various copper and palladium catalysts. Chapter 12 discusses
oxidation reactions. For the most part, these reactions are used for functional group
transformations. A wide variety of reagents are available and we classify them as
based on metals, oxygen and peroxides, and other oxidants. Epoxidation reactions
have special significance in synthesis. The introduction of the epoxide ring can set the
stage for subsequent nucleophilic ring opening to introduce a new group or extend the
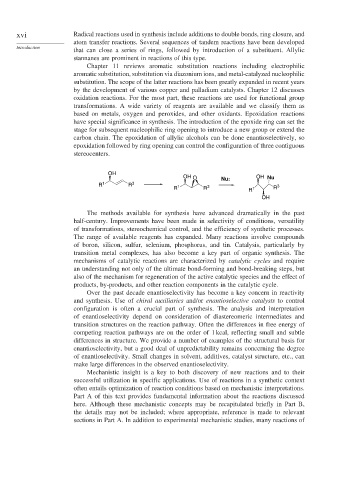
carbon chain. The epoxidation of allylic alcohols can be done enantioselectively, so
epoxidation followed by ring opening can control the configuration of three contiguous
stereocenters.
OH
OH O OH Nu
Nu:
R 1 R 3 3 3
R 1 R R 1 R
OH
The methods available for synthesis have advanced dramatically in the past
half-century. Improvements have been made in selectivity of conditions, versatility
of transformations, stereochemical control, and the efficiency of synthetic processes.
The range of available reagents has expanded. Many reactions involve compounds
of boron, silicon, sulfur, selenium, phosphorus, and tin. Catalysis, particularly by
transition metal complexes, has also become a key part of organic synthesis. The
mechanisms of catalytic reactions are characterized by catalytic cycles and require
an understanding not only of the ultimate bond-forming and bond-breaking steps, but
also of the mechanism for regeneration of the active catalytic species and the effect of
products, by-products, and other reaction components in the catalytic cycle.
Over the past decade enantioselectivity has become a key concern in reactivity
and synthesis. Use of chiral auxiliaries and/or enantioselective catalysts to control
configuration is often a crucial part of synthesis. The analysis and interpretation
of enantioselectivity depend on consideration of diastereomeric intermediates and
transition structures on the reaction pathway. Often the differences in free energy of
competing reaction pathways are on the order of 1 kcal, reflecting small and subtle
differences in structure. We provide a number of examples of the structural basis for
enantioselectivity, but a good deal of unpredictability remains concerning the degree
of enantioselectivity. Small changes in solvent, additives, catalyst structure, etc., can
make large differences in the observed enantioselectivity.
Mechanistic insight is a key to both discovery of new reactions and to their
successful utilization in specific applications. Use of reactions in a synthetic context
often entails optimization of reaction conditions based on mechanistic interpretations.
Part A of this text provides fundamental information about the reactions discussed
here. Although these mechanistic concepts may be recapitulated briefly in Part B,
the details may not be included; where appropriate, reference is made to relevant
sections in Part A. In addition to experimental mechanistic studies, many reactions of