Page 14 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 14
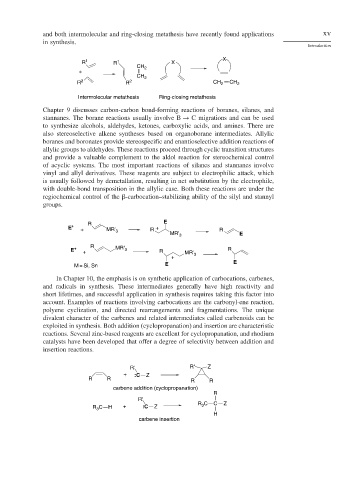
and both intermolecular and ring-closing metathesis have recently found applications xv
in synthesis.
Introduction
X
R 1 R 1 X
CH 2
+
CH 2
R 2 R 2 CH 2 CH 2
Intermolecular metathesis Ring-closing metathesis
Chapter 9 discusses carbon-carbon bond-forming reactions of boranes, silanes, and
stannanes. The borane reactions usually involve B → C migrations and can be used
to synthesize alcohols, aldehydes, ketones, carboxylic acids, and amines. There are
also stereoselective alkene syntheses based on organoborane intermediates. Allylic
boranes and boronates provide stereospecific and enantioselective addition reactions of
allylic groups to aldehydes. These reactions proceed through cyclic transition structures
and provide a valuable complement to the aldol reaction for stereochemical control
of acyclic systems. The most important reactions of silanes and stannanes involve
vinyl and allyl derivatives. These reagents are subject to electrophilic attack, which
is usually followed by demetallation, resulting in net substitution by the electrophile,
with double-bond transposition in the allylic case. Both these reactions are under the
regiochemical control of the -carbocation–stabilizing ability of the silyl and stannyl
groups.
E
R
E + + MR' R + R
3
MR' 3 E
R
E + + MR' 3 R MR' R
+ 3
M = Si, Sn E E
In Chapter 10, the emphasis is on synthetic application of carbocations, carbenes,
and radicals in synthesis. These intermediates generally have high reactivity and
short lifetimes, and successful application in synthesis requires taking this factor into
account. Examples of reactions involving carbocations are the carbonyl-ene reaction,
polyene cyclization, and directed rearrangements and fragmentations. The unique
divalent character of the carbenes and related intermediates called carbenoids can be
exploited in synthesis. Both addition (cyclopropanation) and insertion are characteristic
reactions. Several zinc-based reagents are excellent for cyclopropanation, and rhodium
catalysts have been developed that offer a degree of selectivity between addition and
insertion reactions.
R' R' Z
+ :C Z
R R R R R R
carbene addition (cyclopropanation)
R
R'
C C Z
R 3
R C H + :C Z
3
H
carbene insertion