Page 295 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 295
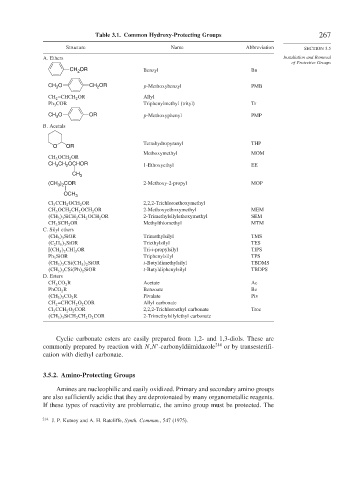
Table 3.1. Common Hydroxy-Protecting Groups 267
Structure Name Abbreviation SECTION 3.5
A. Ethers Installation and Removal
of Protective Groups
CH 2 OR Benzyl Bn
CH O CH OR p-Methoxybenzyl PMB
3
2
CH 2 =CHCH 2 OR Allyl
Ph 3 COR Triphenylmethyl (trityl) Tr
CH O OR p-Methoxyphenyl PMP
3
B. Acetals
Tetrahydropyranyl THP
O OR
Methoxymethyl MOM
CH 3 OCH 2 OR
CH CH OCHOR 1-Ethoxyethyl EE
2
3
CH 3
(CH ) COR 2-Methoxy-2-propyl MOP
3 2
OCH 3
Cl 3 CCH 2 OCH 2 OR 2,2,2-Trichloroethoxymethyl
CH 3 OCH 2 CH 2 OCH 2 OR 2-Methoxyethoxymethyl MEM
CH 3 3 SiCH 2 CH 2 OCH 2 OR 2-Trimethylsilylethoxymethyl SEM
CH 3 SCH 2 OR Methylthiomethyl MTM
C. Silyl ethers
CH 3 3 SiOR Trimethylsilyl TMS
C 2 H 5 3 SiOR Triethylsilyl TES
CH 3 2 CH
3 OR Tri-i-propylsilyl TIPS
Ph 3 SiOR Triphenylsilyl TPS
CH 3 3 CSi CH 3 2 SiOR t-Butyldimethylsilyl TBDMS
CH 3 3 CSi Ph 2 SiOR t-Butyldiphenylsilyl TBDPS
D. Esters
CH 3 CO 2 R Acetate Ac
PhCO 2 R Benzoate Bz
CH 3 3 CO 2 R Pivalate Piv
CH 2 =CHCH 2 O 2 COR Allyl carbonate
Cl 3 CCH 2 O 2 COR 2,2,2-Trichloroethyl carbonate Troc
CH 3 3 SiCH 2 CH 2 O 2 COR 2-Trimethylsilylethyl carbonate
Cyclic carbonate esters are easily prepared from 1,2- and 1,3-diols. These are
commonly prepared by reaction with N,N -carbonyldiimidazole 214 or by transesterifi-
cation with diethyl carbonate.
3.5.2. Amino-Protecting Groups
Amines are nucleophilic and easily oxidized. Primary and secondary amino groups
are also sufficiently acidic that they are deprotonated by many organometallic reagents.
If these types of reactivity are problematic, the amino group must be protected. The
214
J. P. Kutney and A. H. Ratcliffe, Synth. Commun., 547 (1975).