Page 300 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 300
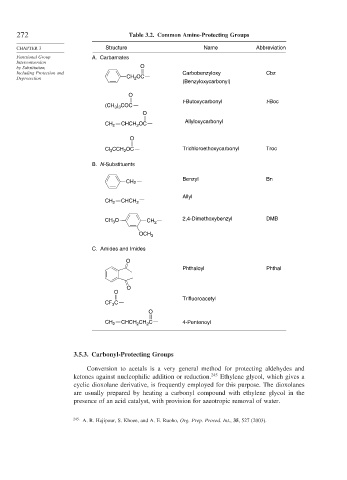
272 Table 3.2. Common Amine-Protecting Groups
CHAPTER 3 Structure Name Abbreviation
Functional Group A. Carbamates
Interconversion
by Substitution, O
Including Protection and Carbobenzyloxy Cbz
Deprotection CH 2 OC
(Benzyloxycarbonyl)
O
t-Butoxycarbonyl t-Boc
(CH ) COC
3 3
O
CH 2 CHCH OC Allyloxycarbonyl
2
O
Cl 3 CCH 2 OC Trichloroethoxycarbonyl Troc
B. N-Substituents
Benzyl Bn
CH 2
Allyl
CH 2 CHCH 2
CH O CH 2 2,4-Dimethoxybenzyl DMB
3
OCH 3
C. Amides and Imides
O
Phthaloyl Phthal
O
O
Trifluoroacetyl
CF C
3
O
CH 2 CHCH CH C 4-Pentenoyl
2
2
3.5.3. Carbonyl-Protecting Groups
Conversion to acetals is a very general method for protecting aldehydes and
ketones against nucleophilic addition or reduction. 245 Ethylene glycol, which gives a
cyclic dioxolane derivative, is frequently employed for this purpose. The dioxolanes
are usually prepared by heating a carbonyl compound with ethylene glycol in the
presence of an acid catalyst, with provision for azeotropic removal of water.
245
A. R. Hajipour, S. Khoee, and A. E. Ruoho, Org. Prep. Proced. Int., 35, 527 (2003).