Page 302 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 302
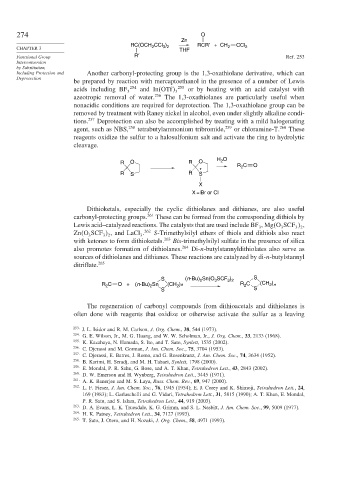
274 O
Zn
RC(OCH CCl ) RCR′ + CH CCl
CHAPTER 3 2 3 2 THF 2 2
R′
Functional Group Ref. 253
Interconversion
by Substitution,
Including Protection and Another carbonyl-protecting group is the 1,3-oxathiolane derivative, which can
Deprotection
be prepared by reaction with mercaptoethanol in the presence of a number of Lewis
acids including BF 3 254 and In OTf 3 255 or by heating with an acid catalyst with
azeotropic removal of water. 256 The 1,3-oxathiolanes are particularly useful when
nonacidic conditions are required for deprotection. The 1,3-oxathiolane group can be
removed by treatment with Raney nickel in alcohol, even under slightly alkaline condi-
tions. 257 Deprotection can also be accomplished by treating with a mild halogenating
agent, such as NBS, 258 tetrabutylammonium tribromide, 259 or chloramine-T. 260 These
reagents oxidize the sulfur to a halosulfonium salt and activate the ring to hydrolytic
cleavage.
O
H 2
R O R O
R 2 C O
+
R S R S
X
X = Br or Cl
Dithioketals, especially the cyclic dithiolanes and dithianes, are also useful
carbonyl-protecting groups. 261 These can be formed from the corresponding dithiols by
Lewis acid–catalyzed reactions. The catalysts that are used include BF ,Mg O SCF ,
3
3 2
3
Zn O SCF , and LaCl . 262 S-Trimethylsilyl ethers of thiols and dithiols also react
3
3 2
3
with ketones to form dithioketals. 263 Bis-trimethylsilyl sulfate in the presence of silica
also promotes formation of dithiolanes. 264 Di-n-butylstannyldithiolates also serve as
sources of dithiolanes and dithianes. These reactions are catalyzed by di-n-butylstannyl
ditriflate. 265
S (n-Bu) 2 Sn(O 3 SCF 3 ) 2 S
R C O + (n-Bu) Sn (CH )n R C (CH )
2 n
2
2
2
2
S S
The regeneration of carbonyl compounds from dithioacetals and dithiolanes is
often done with reagents that oxidize or otherwise activate the sulfur as a leaving
253 J. L. Isidor and R. M. Carlson, J. Org. Chem., 38, 544 (1973).
254
G. E. Wilson, Jr., M. G. Huang, and W. W. Scholman, Jr., J. Org. Chem., 33, 2133 (1968).
255
K. Kazahaya, N. Hamada, S. Ito, and T. Sato, Synlett, 1535 (2002).
256 C. Djerassi and M. Gorman, J. Am. Chem. Soc., 75, 3704 (1953).
257 C. Djerassi, E. Batres, J. Romo, and G. Rosenkranz, J. Am. Chem. Soc., 74, 3634 (1952).
258
B. Karimi, H. Seradj, and M. H. Tabaei, Synlett, 1798 (2000).
259
E. Mondal, P. R. Sahu, G. Bose, and A. T. Khan, Tetrahedron Lett., 43, 2843 (2002).
260 D. W. Emerson and H. Wynberg, Tetrahedron Lett., 3445 (1971).
261
A. K. Banerjee and M. S. Laya, Russ. Chem. Rev., 69, 947 (2000).
262
L. F. Fieser, J. Am. Chem. Soc., 76, 1945 (1954); E. J. Corey and K. Shimoji, Tetrahedron Lett., 24,
169 (1983); L. Garlaschelli and G. Vidari, Tetrahedron Lett., 31, 5815 (1990); A. T. Khan, E. Mondal,
P. R. Satu, and S. Islam, Tetrahedron Lett., 44, 919 (2003).
263 D. A. Evans, L. K. Truesdale, K. G. Grimm, and S. L. Nesbitt, J. Am. Chem. Soc., 99, 5009 (1977).
264 H. K. Patney, Tetrahedron Lett., 34, 7127 (1993).
265
T. Sato, J. Otero, and H. Nozaki, J. Org. Chem., 58, 4971 (1993).