Page 307 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 307
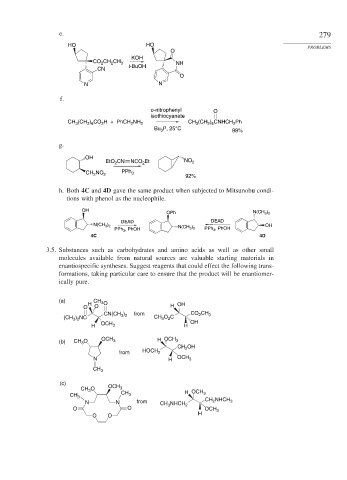
e. 279
HO HO PROBLEMS
O
KOH
CO 2 CH CH 3 NH
2
CN t-BuOH
O
N N
f.
o-nitrophenyl O
isothiocyanate
CH (CH ) CO H + PhCH NH 2 CH (CH ) CNHCH Ph
2
2 6
2
2
2 6
3
3
P, 25°C
Bu 3
99%
g.
OH
EtO CN NCO Et NO 2
2
2
CH NO 2 PPh 3 92%
2
h. Both 4C and 4D gave the same product when subjected to Mitsunobu condi-
tions with phenol as the nucleophile.
OH
OPh N(CH 3 ) 2
DEAD DEAD
N(CH 3 ) 2 OH
PPh 3 , PhOH N(CH 3 ) 2 PPh 3 , PhOH
4C 4D
3.5. Substances such as carbohydrates and amino acids as well as other small
molecules available from natural sources are valuable starting materials in
enantiospecific syntheses. Suggest reagents that could effect the following trans-
formations, taking particular care to ensure that the product will be enantiomer-
ically pure.
(a) CH 3 O
O H O H OH
CN(CH ) from CO CH 3
2
(CH ) NC 3 2 CH 3 O 2 C
3 2
H OCH 3 H OH
(b) CH O OCH 3 H OCH 3
3
CH OH
2
from HOCH 2
N H OCH 3
CH 3
(c) OCH
CH O 3
3
CH 3 CH 3 H OCH 3
2
N N from CH NHCH 2 CH NHCH 3
3
O O OCH 3
O O H