Page 311 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 311
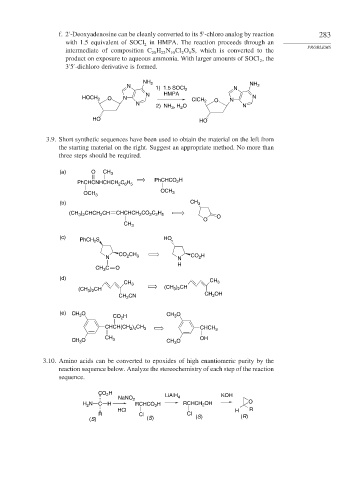
f. 2 -Deoxyadenosine can be cleanly converted to its 5 -chloro analog by reaction 283
with 1.5 equivalent of SOCl in HMPA. The reaction proceeds through an PROBLEMS
2
intermediate of composition C H N Cl O S, which is converted to the
10
22
5
2
20
product on exposure to aqueous ammonia. With larger amounts of SOCl , the
2
3 5 -dichloro derivative is formed.
NH 2 NH
N 2
1) 1.5 SOCl 2 N
N HMPA
HOCH 2 O N ClCH O N N
N 2
2) NH , H O N
3
2
HO HO
3.9. Short synthetic sequences have been used to obtain the material on the left from
the starting material on the right. Suggest an appropriate method. No more than
three steps should be required.
(a) O CH 3
PhCHCNHCHCH C H PhCHCO 2 H
2 6 5
OCH
OCH 3 3
(b) CH 3
(CH ) CHCH CH CHCHCH 2 CO C H
2 2 5
3 2
2
O O
CH 3
(c) HO
PhCH S
2
CH
N CO 2 3 N CO H
2
H
CH C O
3
(d) CH
CH 3 3
) CH (CH ) CH
3 2
(CH 3 2
CH OH
CH CN 2
2
(e) CH O CO H CH O
3
3
2
CHCH(CH ) CH 3 CHCH 3
2 4
CH OH
CH O 3 CH O
3
3
3.10. Amino acids can be converted to epoxides of high enantiomeric purity by the
reaction sequence below. Analyze the stereochemistry of each step of the reaction
sequence.
H
CO 2 LiAlH KOH
NaNO 2 4
H N C H RCHCO H RCHCH OH O
2
2
2
HCl H R
R Cl Cl (R)
(S) (S) (S)