Page 314 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 314
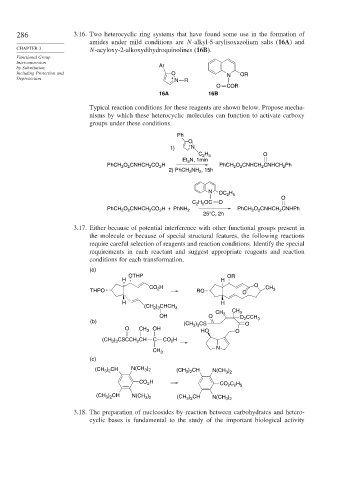
286 3.16. Two heterocyclic ring systems that have found some use in the formation of
amides under mild conditions are N-alkyl-5-arylisoxazolium salts (16A) and
CHAPTER 3 N-acyloxy-2-alkoxydihydroquinolines (16B).
Functional Group
Interconversion
by Substitution, Ar
Including Protection and O N OR
Deprotection + N R
O COR
16A 16B
Typical reaction conditions for these reagents are shown below. Propose mecha-
nisms by which these heterocyclic molecules can function to activate carboxy
groups under these conditions.
Ph
O
+
1) N
O
C 2 H 5
N, 1min
Et 3
PhCH O CNHCH CO H PhCH O CNHCH CNHCH Ph
2
2
2
2
2
2
2
2
2) PhCH NH , 15h
2
2
N OC H
2 5
O
C H OC O
2 5
PhCH O CNHCH CO H + PhNH 2 PhCH O CNHCH CNHPh
2
2
2
2
2
2
2
25°C, 2h
3.17. Either because of potential interference with other functional groups present in
the molecule or because of special structural features, the following reactions
require careful selection of reagents and reaction conditions. Identify the special
requirements in each reactant and suggest appropriate reagents and reaction
conditions for each transformation.
(a)
OTHP OR
H H
H O
CO 2 CH 3
THPO RO O
H H
(CH ) CHCH 3
2 3
CH CH 3
OH O 3 O CCH
(b) (CH ) CS 2 O 3
O CH 3 OH 3 3 HO O
(CH 3 ) 3 CSCCH 2 CH C CO H
2
N
CH 3
(c)
(CH ) CH N(CH ) (CH ) CH N(CH )
3 2
3 2
3 2
3 2
CO H CO C H
2
2 2 5
(CH ) CH N(CH ) (CH ) CH N(CH )
3 2
3 2
3 2
3 2
3.18. The preparation of nucleosides by reaction between carbohydrates and hetero-
cyclic bases is fundamental to the study of the important biological activity