Page 316 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 316
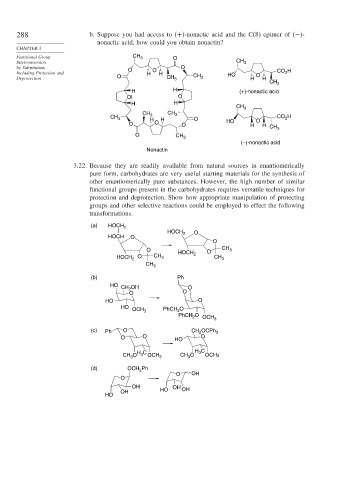
288 b. Suppose you had access to (+)-nonactic acid and the C(8) epimer of (−)-
nonactic acid, how could you obtain nonactin?
CHAPTER 3
Functional Group CH 3 O
Interconversion CH 3
by Substitution, O O O
Including Protection and H H HO O CO 2 H
Deprotection O CH 3 CH 3 H H
CH 3
H H (+)-nonactic acid
OI O
H H
CH 3
CH 3 CH 3
H H HO O
CH 3 O CO 2 H
O O O H H CH 3
O CH 3
(–)-nonactic acid
Nonactin
3.22. Because they are readily available from natural sources in enantiomerically
pure form, carbohydrates are very useful starting materials for the synthesis of
other enantiomerically pure substances. However, the high number of similar
functional groups present in the carbohydrates requires versatile techniques for
protection and deprotection. Show how appropriate manipulation of protecting
groups and other selective reactions could be employed to effect the following
transformations.
(a) HOCH 2
HOCH 2 O
HOCH O
O
O HOCH O CH 3
HOCH 2 O CH 3 2 CH 3
CH 3
(b) Ph
HO
CH 2 OH O
O O
HO O
HO O
OCH 3 PhCH 2
PhCH 2 O
OCH 3
(c) Ph O CH 2 OCPh 3
O O HO O
H C H C
3
O O
CH 3 3 OCH 3 CH 3 OCH3
(d) OCH Ph
2
O OH
O
OH OH
OH HO OH
HO