Page 313 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 313
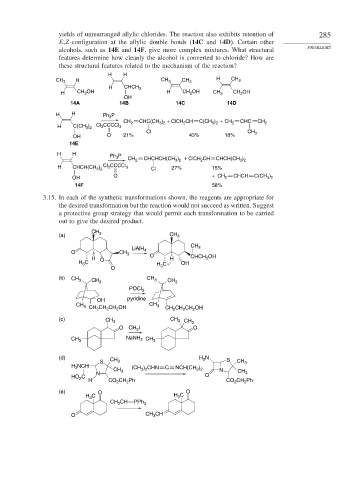
yields of unrearranged allylic chlorides. The reaction also exhibits retention of 285
E,Z-configuration at the allylic double bonds (14C and 14D). Certain other
alcohols, such as 14E and 14F, give more complex mixtures. What structural PROBLEMS
features determine how cleanly the alcohol is converted to chloride? How are
these structural features related to the mechanism of the reaction?
H H
CH 3 H CH 3 CH 3 H CH 3
H CHCH 3
H CH 2 OH H CH 2 OH CH 3 CH 2 OH
OH
14A 14B 14C 14D
H H Ph 3 P
+ ClCH 2 CH CHC
CH 2 CHC(CH 3 ) 2 C(CH 3 ) 2 + CH 2 CH 2
H C(CH 3 ) 2 Cl 3 CCCCl 3
Cl CH 3
OH O 21% 43% 18%
14E
H H Ph 3 P
CH 2 CHCHCH(CH 3 ) 2 + ClCH 2 CH CHCH(CH 3 ) 2
H CHCH(CH 3 ) 2 Cl 3 CCCCl 3 Cl 27% 15%
O CHCH
OH + CH 2 C(CH 3 ) 2
14F 58%
3.15. In each of the synthetic transformations shown, the reagents are appropriate for
the desired transformation but the reaction would not succeed as written. Suggest
a protective group strategy that would permit each transformation to be carried
out to give the desired product.
CH
(a) 3 CH 3
LiAlH CH 3
O CH 3 4
H O O H CHCH 2 OH
C
H 3 C OH
H 3
O
(b) CH 3 CH 3 CH 3 CH 3
POCl 3
OH pyridine
CH 3 CH 3
CH 2 CH 2 CH 2 OH CH CH CH OH
2
2
2
(c) CH 3 CH 3 CH 3
O CH I O
3
CH 3 NaNH CH 3
2
(d) CH H N
2
S 3 S CH 3
H 2 NCH (CH ) CHN C NCH(CH )
CH 3 2 3 2 N CH
N 3 O 3
HO 2 C
H CO 2 CH Ph CO CH Ph
2
2
2
(e) O O
H 3 C H 3 C
CH CH PPh 3
3
O CH 3 CH