Page 377 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 377
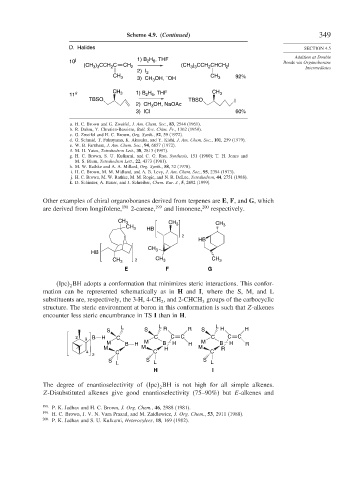
Scheme 4.9. (Continued) 349
D. Halides SECTION 4.5
Addition at Double
10 j 1) B H , THF Bonds via Organoborane
2 6
(CH ) CCH C CH 2 (CH ) CCH CHCH I Intermediates
2
2
3 3
3 3
2
2) I 2
CH 3 3) CH OH, OH CH 3 92%
–
3
11 k CH 3 1) B H , THF CH 3
2 6
TBSO TBSO I
2) CH OH, NaOAc
3
3) ICl 60%
a. H. C. Brown and G. Zweifel, J. Am. Chem. Soc., 83, 2544 (1961).
b. R. Dulou, Y. Chretien-Bessiere, Bull. Soc. Chim. Fr., 1362 (1959).
c. G. Zweifel and H. C. Brown, Org. Synth., 52, 59 (1972).
d. G. Schmid, T. Fukuyama, K. Akasaka, and Y. Kishi, J. Am. Chem. Soc., 101, 259 (1979).
e. W. B. Farnham, J. Am. Chem. Soc., 94, 6857 (1972).
f. M. H. Yates, Tetrahedron Lett., 38, 2813 (1997).
g. H. C. Brown, S. U. Kulkarni, and C. G. Rao, Synthesis, 151 (1980); T. H. Jones and
M. S. Blum, Tetrahedron Lett., 22, 4373 (1981).
h. M. W. Rathke and A. A. Millard, Org. Synth., 58, 32 (1978).
i. H. C. Brown, M. M. Midland, and A. B. Levy, J. Am. Chem. Soc., 95, 2394 (1973).
j. H. C. Brown, M. W. Rathke, M. M. Rogic, and N. R. DeLue, Tetrahedron, 44, 2751 (1988).
k. D. Schinzer, A. Bauer, and J. Schreiber, Chem. Eur. J., 5, 2492 (1999).
Other examples of chiral organoboranes derived from terpenes are E, F, and G, which
are derived from longifolene, 198 2-carene, 199 and limonene, 200 respectively.
CH 3 CH CH
CH 3 HB 3 3
2
HB
CH
HB 3
CH 3 2 CH 3 CH 3
E F G
Ipc BH adopts a conformation that minimizes steric interactions. This confor-
2
mation can be represented schematically as in H and I, where the S, M, and L
substituents are, respectively, the 3-H, 4-CH , and 2-CHCH groups of the carbocyclic
2 3
structure. The steric environment at boron in this conformation is such that Z-alkenes
encounter less steric encumbrance in TS I than in H.
L L L
S S R R S H H
2 B H C C C C C C C
3
M B H M B H H M B H R
M M H M R
4 C C C
2
S S S
L L L
H I
The degree of enantioselectivity of Ipc BH is not high for all simple alkenes.
2
Z-Disubstituted alkenes give good enantioselectivity (75–90%) but E-alkenes and
198
P. K. Jadhav and H. C. Brown, J. Org. Chem., 46, 2988 (1981).
199 H. C. Brown, J. V. N. Vara Prasad, and M. Zaidlewicz, J. Org. Chem., 53, 2911 (1988).
200
P. K. Jadhav and S. U. Kulkarni, Heterocylces, 18, 169 (1982).