Page 379 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 379
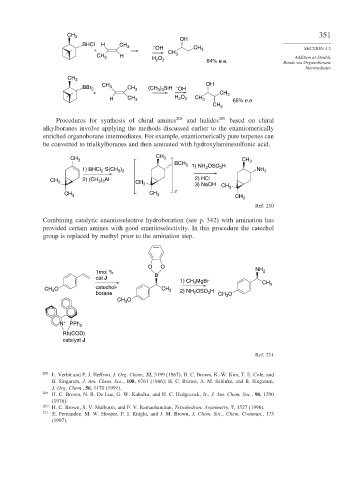
CH 3 351
OH
BHCl H CH 3 –
+ OH CH CH 3 SECTION 4.5
H 3
Addition at Double
CH 3 H O 2 64% e.e. Bonds via Organoborane
2
Intermediates
CH 3
CH OH
BBr 2 3 CH 3 (CH ) SiH – OH
3 3
+ CH 3
H CH 3 H 2 O 2 CH 3 65% e.e.
CH 3
Procedures for synthesis of chiral amines 208 and halides 209 based on chiral
alkylboranes involve applying the methods discussed earlier to the enantiomerically
enriched organoborane intermediates. For example, enantiomerically pure terpenes can
be converted to trialkylboranes and then aminated with hydroxylaminesulfonic acid.
CH 3 CH 3 CH 3
. BCH 3 1) NH OSO H
2
3
1) BHCl S(CH ) NH 2
2
3 2
CH 3 2) (CH ) AI CH 3 2) HCl CH 3
3 3
3) NaOH
CH 3 CH 3 2 CH 3
Ref. 210
Combining catalytic enantioselective hydroboration (see p. 342) with amination has
provided certain amines with good enantioselectivity. In this procedure the catechol
group is replaced by methyl prior to the amination step.
O O
1mol % NH 2
cat J B
1) CH MgBr CH 3
3
CH O catechol- CH 3 2) NH OSO H
3
borane 2 3 CH O
3
CH O
3
N + PPh 2
Rh(COD)
catalyst J
Ref. 211
208 L. Verbit and P. J. Heffron, J. Org. Chem., 32, 3199 (1967); H. C. Brown, K.-W. Kim, T. E. Cole, and
B. Singaram, J. Am. Chem. Soc., 108, 6761 (1986); H. C. Brown, A. M. Sahinke, and B. Singaram,
J. Org. Chem., 56, 1170 (1991).
209
H. C. Brown, N. R. De Lue, G. W. Kabalka, and H. C. Hedgecock, Jr., J. Am. Chem. Soc., 98, 1290
(1976).
210 H. C. Brown, S. V. Malhotra, and P. V. Ramachandran, Tetrahedron: Asymmetry, 7, 3527 (1996).
211
E. Fernandez, M. W. Hooper, F. I. Knight, and J. M. Brown, J. Chem. Soc., Chem. Commun., 173
(1997).