Page 384 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 384
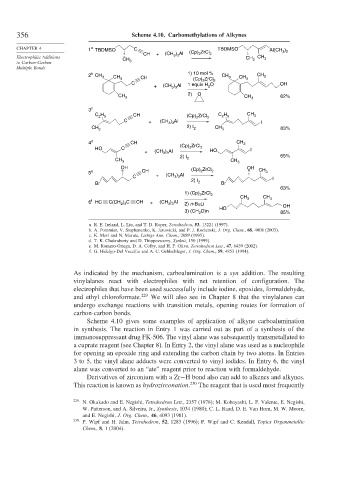
356 Scheme 4.10. Carbomethylations of Alkynes
a
CHAPTER 4 1 TBDMSO C TBDMSO Al(CH )
CH + (CH ) Al (Cp) ZrCl 2 3 2
2
Electrophilic Additions CH 3 3 CH CH 3
3
to Carbon-Carbon 3
Multiple Bonds
b
2 CH 1) 10 mol % CH CH
3 CH 3 CH (Cp) ZrCl 3 CH 3 3
2
C 1 equiv H O 2 OH
+ (CH ) Al 2
3 3
2) O
CH 3 CH 3 62%
3 c
C H CH (Cp) ZrCl C H CH 3
2 5
2 5
C + (CH 3 3 2 2 I
) Al
CH 3 2) I 2 CH 3 83%
4 d CH CH
(Cp) ZrCl 3
HO C 2 2 I
+ (CH ) Al HO
3 3
2) I 65%
CH 3 2 CH 3
OH (Cp) ZrCl OH CH
5 e CH 2 2 3
C + (CH ) Al I
3 3
2) I
Br 2 Br
63%
1) (Cp) ZrCl 2
2
CH 3 CH 3
f
) C
6 HC C(CH 2 3 CH + (CH ) Al 2) n-BuLi
3 3
HO OH
O)n
3) (CH 2 85%
a. R. E. Ireland, L. Liu, and T. D. Roper, Tetrahedron, 53, 13221 (1997).
b. A. Pommier, V. Stephanenko, K. Jarowicki, and P. J. Kocienski, J. Org. Chem., 68, 4008 (2003).
c. K. Mori and N. Murata, Liebigs Ann. Chem., 2089 (1995).
d. T. K. Chakraborty and D. Thippeswamy, Synlett, 150 (1999).
e. M. Romero-Ortega, D. A. Colby, and H. F. Olivo, Tetrahedron Lett., 47, 6439 (2002).
f. G. Hidalgo-Del Vecchio and A. C. Oehlschlager, J. Org. Chem.,59, 4853 (1994).
As indicated by the mechanism, carboalumination is a syn addition. The resulting
vinylalanes react with electrophiles with net retention of configuration. The
electrophiles that have been used successfully include iodine, epoxides, formaldehyde,
and ethyl chloroformate. 229 We will also see in Chapter 8 that the vinylalanes can
undergo exchange reactions with transition metals, opening routes for formation of
carbon-carbon bonds.
Scheme 4.10 gives some examples of application of alkyne carboalumination
in synthesis. The reaction in Entry 1 was carried out as part of a synthesis of the
immunosuppressant drug FK-506. The vinyl alane was subsequently transmetallated to
a cuprate reagent (see Chapter 8). In Entry 2, the vinyl alane was used as a nucleophile
for opening an epoxide ring and extending the carbon chain by two atoms. In Entries
3 to 5, the vinyl alane adducts were converted to vinyl iodides. In Entry 6, the vinyl
alane was converted to an “ate” reagent prior to reaction with formaldehyde.
Derivatives of zirconium with a Zr−H bond also can add to alkenes and alkynes.
This reaction is known as hydrozirconation. 230 The reagent that is used most frequently
229 N. Okukado and E. Negishi, Tetrahedron Lett., 2357 (1978); M. Kobayashi, L. F. Valente, E. Negishi,
W. Patterson, and A. Silveira, Jr., Synthesis, 1034 (1980); C. L. Rand, D. E. Van Horn, M. W. Moore,
and E. Negishi, J. Org. Chem., 46, 4093 (1981).
230
P. Wipf and H. Jahn, Tetrahedron, 52, 1283 (1996); P. Wipf and C. Kendall, Topics Organmetallic
Chem., 8, 1 (2004).