Page 388 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 388
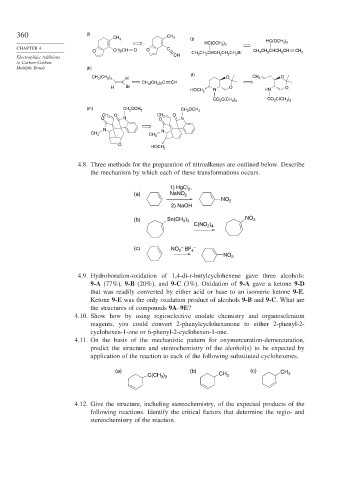
360 (i)
CH 3 CH 3 (j)
HC(OCH 3 ) 2
HC(OCH 3 ) 2
CHAPTER 4 O O C
O CH 2 CH CH 3 CH 2 CHCH 2 CH 2 CH 2 Br CH 3 CH 2 CHCH 2 CH CH 2
CH
Electrophilic Additions
to Carbon-Carbon
Multiple Bonds (k)
(l)
CH 3 (CH 2 ) 5 H O CH 2 O
CH 3 (CH 2 ) 5 C CH
H Br O O
HOCH 2 N HN
CO 2 C(CH 3 ) 3 CO 2 C(CH 3 ) 3
(m) CH 2 OCH 3 CH 2 OCH 3
CH 2 O CH 2 O
O N O N
N N
CH 3
CH 3
O
HOCH 2
4.8. Three methods for the preparation of nitroalkenes are outlined below. Describe
the mechanism by which each of these transformations occurs.
1) HgCl ,
2
(a) NaNO 2
NO 2
2) NaOH
(b) Sn(CH ) NO 2
3 3
C(NO )
2 4
(c) NO 2 + BF 4 –
NO 2
4.9. Hydroboration-oxidation of 1,4-di-t-butylcyclohexene gave three alcohols:
9-A (77%), 9-B (20%), and 9-C (3%). Oxidation of 9-A gave a ketone 9-D
that was readily converted by either acid or base to an isomeric ketone 9-E.
Ketone 9-E was the only oxidation product of alcohols 9-B and 9-C. What are
the structures of compounds 9A–9E?
4.10. Show how by using regioselective enolate chemistry and organoselenium
reagents, you could convert 2-phenylcyclohexanone to either 2-phenyl-2-
cyclohexen-1-one or 6-phenyl-2-cyclohexen-1-one.
4.11. On the basis of the mechanistic pattern for oxymercuration-demercuration,
predict the structure and stereochemistry of the alcohol(s) to be expected by
application of the reaction to each of the following substituted cyclohexenes.
(a) (b) (c) CH
C(CH ) CH 3 3
3 3
4.12. Give the structure, including stereochemistry, of the expected products of the
following reactions. Identify the critical factors that determine the regio- and
stereochemistry of the reaction.