Page 391 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 391
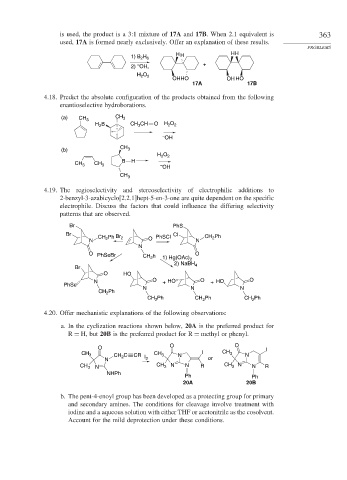
is used, the product is a 3:1 mixture of 17A and 17B. When 2.1 equivalent is 363
used, 17A is formed nearly exclusively. Offer an explanation of these results.
PROBLEMS
1) B H H H HH
2 6
–
2) OH, +
O
H 2 2
OHHO OH HO
17A 17B
4.18. Predict the absolute configuration of the products obtained from the following
enantioselective hydroborations.
(a) CH 3 CH 3
H B CH CH O H O 2
2
3
2
– OH
(b) CH 3
H O 2
2
B H
CH 3 CH 3 – OH
CH 3
4.19. The regioselectivity and stereoselectivity of electrophilic additions to
2-benzyl-3-azabicyclo[2.2.1]hept-5-en-3-one are quite dependent on the specific
electrophile. Discuss the factors that could influence the differing selectivity
patterns that are observed.
Br PhS
Br CH Ph Br PhSCl Cl CH Ph
N 2 2 O N 2
N
O PhSeBr O
CH h 1) Hg(OAc) 2
2
2) NaBH
Br 4
O HO
N O + HO O + HO O
PhSe N N N
CH Ph
2
CH Ph CH Ph CH Ph
2
2
2
4.20. Offer mechanistic explanations of the following observations:
a. In the cyclization reactions shown below, 20A is the preferred product for
R = H, but 20B is the preferred product for R = methyl or phenyl.
O O O I
CH C CR 3 N N
CH 3 CH I CH 3
2
N I 2 or
CH 3 N CH 3 N N R CH 3 N N R
NHPh
Ph Ph
20A 20B
b. The pent-4-enoyl group has been developed as a protecting group for primary
and secondary amines. The conditions for cleavage involve treatment with
iodine and a aqueous solution with either THF or acetonitrile as the cosolvent.
Account for the mild deprotection under these conditions.