Page 390 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 390
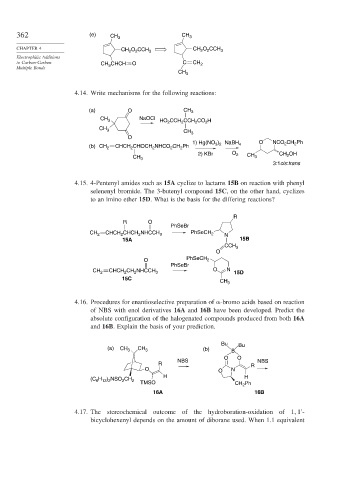
362 (e) CH 3 CH 3
CHAPTER 4 CH O CCH 3 CH O CCH 3
2
2
2
2
Electrophilic Additions
to Carbon-Carbon CH CHCH O C CH 2
Multiple Bonds 3
CH 3
4.14. Write mechanisms for the following reactions:
(a) O CH 3
CH 3 NaOCl HO CCH CCH CO H
2
2
2
2
CH 3 CH 3
O
1) Hg(NO ) NaBH O NCO CH Ph
(b) CH 2 CHCH CHOCH NHCO CH Ph 3 2 4 2 2
2
2
2
2
2) KBr O 2 CH CH 2 OH
CH 3 3
3:1cis:trans
4.15. 4-Pentenyl amides such as 15A cyclize to lactams 15B on reaction with phenyl
selenenyl bromide. The 3-butenyl compound 15C, on the other hand, cyclizes
to an imino ether 15D. What is the basis for the differing reactions?
R
R O
PhSeBr
CH 2 CHCH 2 CHCH NHCCH 3 PhSeCH 2 N
2
15A 15B
CCH 3
O
O PhSeCH 2
PhSeBr
CH 2 CHCH CH NHCCH 3 O N 15D
2
2
15C CH 3
4.16. Procedures for enantioselective preparation of -bromo acids based on reaction
of NBS with enol derivatives 16A and 16B have been developed. Predict the
absolute configuration of the halogenated compounds produced from both 16A
and 16B. Explain the basis of your prediction.
Bu Bu
(a) CH 3 CH 3 (b) B
O O
NBS NBS
R R
O O N
H H
(C H ) NSO CH
2
6 13 2
2
TMSO CH Ph
2
16A 16B
4.17. The stereochemical outcome of the hydroboration-oxidation of 1 1 -
bicyclohexenyl depends on the amount of diborane used. When 1.1 equivalent