Page 393 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 393
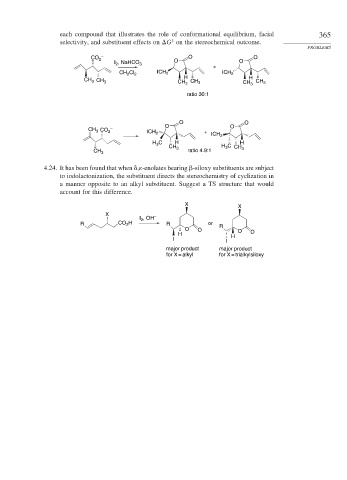
each compound that illustrates the role of conformational equilibrium, facial 365
‡
selectivity, and substituent effects on G on the stereochemical outcome.
PROBLEMS
CO 2 – O O
I , NaHCO 3 O O
2
+
CH Cl 2 ICH 2 ICH 2
2
H
CH 3 CH 3 CH CH 3 CH H 3 CH 3
3
ratio 30:1
O O
O O
CH 3 CO 2 – ICH 2 + ICH 2
H C H H
3
CH 3 H C CH
3
CH 3 ratio 4.9:1 3
4.24. It has been found that when , -enolates bearing -siloxy substituents are subject
to iodolactonization, the substituent directs the stereochemistry of cyclization in
a manner opposite to an alkyl substituent. Suggest a TS structure that would
account for this difference.
X X
X –
I , OH
2
R CO 2 H R or R
O O O
H H O
I I
major product major product
for X = alkyl for X = trialkylsiloxy