Page 397 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 397
370 implied by the above mechanism, the intermediate must remain bonded to the metal
surface in such a way that the stereochemical relationship is maintained. Adsorption to
CHAPTER 5 the catalyst surface normally involves the less sterically congested side of the double
Reduction of bond, and as a result hydrogen is added from the less hindered face of the double
Carbon-Carbon Multiple
Bonds, Carbonyl bond. There are many hydrogenations in which hydrogen addition is not entirely syn,
Groups, and Other and independent corroboration of the stereochemistry is normally necessary.
Functional Groups
Scheme 5.1 illustrates some hydrogenations in which the syn addition from the
less hindered side is observed. Some exceptions are also included. Entry 1 shows the
hydrogenation of an exocyclic methylene group. This reaction was studied at various
H pressures and over both Pt and Pd catalysts. 4-Methyl- and 4-t-butylmethylene
2
3
cyclohexane also give mainly the cis product. These results are consistent with a
favored (2.3:1) equatorial delivery of hydrogen.
CH 3 CH 3
CH 2 H
H CH 2 CH 3 CH 3
H H
H H H
H
H
H
The Entry 2 reactant, 1,2-dimethylcyclohexene, was also studied by several groups
and a 2:1– 4:1 preference for syn addition was noted, depending on the catalyst and
conditions. In the reference cited, the catalyst was prepared by reduction of a Pt salt
with NaBH . A higher ratio of the cis product was noted at 0 C (5.2:1) than at 25 C
4
(2.5:1). In Entry 3, the 2,6-dimethycyclohexene gives mainly cis product with a Pt
catalyst but trans product dominates with a Pd catalyst. These three cases indicate
that stereoselectivity for unhindered alkenes is modest and dependent on reaction
conditions. Entries 4 and 5 involve more rigid and sterically demanding alkenes. In
both cases, syn addition of hydrogen occurs from the less hindered face of the molecule.
Entries 6 to 8 are cases in which hydrogen is added from the more-substituted face
of the double bond. The compound in Entry 6 gives mainly trans product at high H 2
pressure, where the effects of alkene isomerization are minimized. This result indicates
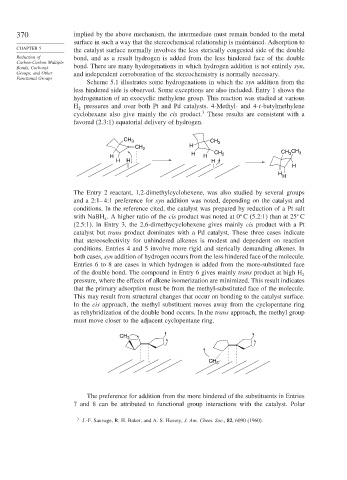
that the primary adsorption must be from the methyl-substituted face of the molecule.
This may result from structural changes that occur on bonding to the catalyst surface.
In the cis approach, the methyl substituent moves away from the cyclopentane ring
as rehybridization of the double bond occurs. In the trans approach, the methyl group
must move closer to the adjacent cyclopentane ring.
CH 3
CH 3
The preference for addition from the more hindered of the substituents in Entries
7 and 8 can be attributed to functional group interactions with the catalyst. Polar
3
J.-F. Sauvage, R. H. Baker, and A. S. Hussey, J. Am. Chem. Soc., 82, 6090 (1960).