Page 399 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 399
372 groups sometimes favor cis addition of hydrogen, relative to the substituent. This is a
very common observation for hydroxy groups, but less so for esters (vide infra).
CHAPTER 5
The facial stereoselectivity of hydrogenation is affected by the presence of polar
Reduction of functional groups that can govern the mode of adsorption to the catalyst surface. For
Carbon-Carbon Multiple
Bonds, Carbonyl instance, there are many of examples of hydrogen being introduced from the face of
Groups, and Other
Functional Groups the molecule occupied by the hydroxy group, which indicates that the hydroxy group
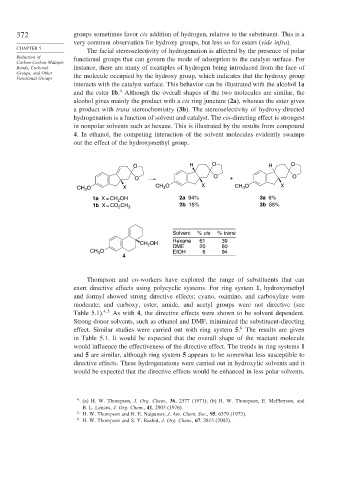
interacts with the catalyst surface. This behavior can be illustrated with the alcohol 1a
4
and the ester 1b. Although the overall shapes of the two molecules are similar, the
alcohol gives mainly the product with a cis ring juncture (2a), whereas the ester gives
a product with trans stereochemistry (3b). The stereoselectivity of hydroxy-directed
hydrogenation is a function of solvent and catalyst. The cis-directing effect is strongest
in nonpolar solvents such as hexane. This is illustrated by the results from compound
4. In ethanol, the competing interaction of the solvent molecules evidently swamps
out the effect of the hydroxymethyl group.
O H O H O
O O + O
3
CH O X CH 3 O X CH O X
3
1a X = CH OH 2a 94% 3a 6%
2
1b X = CO CH 3 2b 15% 3b 85%
2
Solvent % cis % trans
Hexane 61 39
CH OH DME 20 80
2
O
CH 3 EtOH 6 94
4
Thompson and co-workers have explored the range of substituents that can
exert directive effects using polycyclic systems. For ring system 1, hydroxymethyl
and formyl showed strong directive effects; cyano, oximino, and carboxylate were
moderate; and carboxy, ester, amide, and acetyl groups were not directive (see
Table 5.1). 4 5 As with 4, the directive effects were shown to be solvent dependent.
Strong donor solvents, such as ethanol and DMF, minimized the substituent-directing
6
effect. Similar studies were carried out with ring system 5. The results are given
in Table 5.1. It would be expected that the overall shape of the reactant molecule
would influence the effectiveness of the directive effect. The trends in ring systems 1
and 5 are similar, although ring system 5 appears to be somewhat less susceptible to
directive effects. These hydrogenations were carried out in hydroxylic solvents and it
would be expected that the directive effects would be enhanced in less polar solvents.
4 (a) H. W. Thompson, J. Org. Chem., 36, 2577 (1971); (b) H. W. Thompson, E. McPherson, and
B. L. Lences, J. Org. Chem., 41, 2903 (1976).
5 H. W. Thompson and R. E. Naipawer, J. Am. Chem. Soc., 95, 6379 (1973).
6
H. W. Thompson and S. Y. Rashid, J. Org. Chem., 67, 2813 (2002).