Page 402 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 402
375
CH 3
O
SECTION 5.1
PhP PPh 2 Addition of Hydrogen at
Rh + Carbon-Carbon Multiple
CH O C O Bonds
3
2
PhCH 2 N
CH 3
H
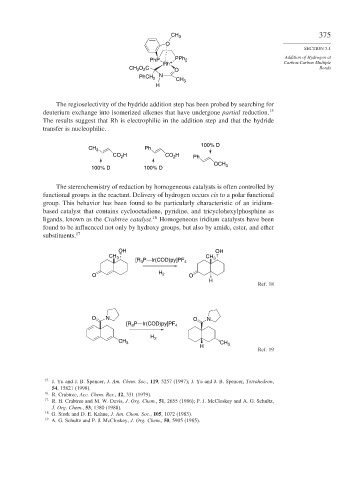
The regioselectivity of the hydride addition step has been probed by searching for
deuterium exchange into isomerized alkenes that have undergone partial reduction. 15
The results suggest that Rh is electrophilic in the addition step and that the hydride
transfer is nucleophilic.
100% D
CH 3 Ph
CO H CO H Ph
2
2
OCH
100% D 100% D 3
The stereochemistry of reduction by homogeneous catalysts is often controlled by
functional groups in the reactant. Delivery of hydrogen occurs cis to a polar functional
group. This behavior has been found to be particularly characteristic of an iridium-
based catalyst that contains cyclooctadiene, pyridine, and tricyclohexylphosphine as
ligands, known as the Crabtree catalyst. 16 Homogeneous iridium catalysts have been
found to be influenced not only by hydroxy groups, but also by amide, ester, and ether
substituents. 17
OH OH
CH 3 CH 3
[R P—Ir(COD)py]PF 4
3
O H 2 O
H
Ref. 18
O N O N
[R P—Ir(COD)py]PF 4
3
H 2
CH 3 CH 3
H
Ref. 19
15 J. Yu and J. B. Spencer, J. Am. Chem. Soc., 119, 5257 (1997); J. Yu and J. B. Spencer, Tetrahedron,
54, 15821 (1998).
16 R. Crabtree, Acc. Chem. Res., 12, 331 (1979).
17
R. H. Crabtree and M. W. Davis, J. Org. Chem., 51, 2655 (1986); P. J. McCloskey and A. G. Schultz,
J. Org. Chem., 53, 1380 (1988).
18 G. Stork and D. E. Kahne, J. Am. Chem. Soc., 105, 1072 (1983).
19
A. G. Schultz and P. J. McCloskey, J. Org. Chem., 50, 5905 (1985).