Page 400 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 400
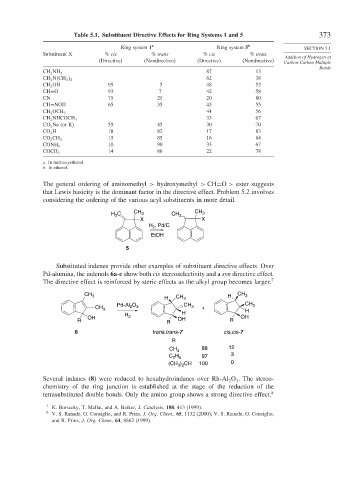
Table 5.1. Substituent Directive Effects for Ring Systems 1 and 5 373
Ring system 1 a Ring system 5 b SECTION 5.1
Substituent X % cis % trans % cis % trans
Addition of Hydrogen at
(Directive) (Nondirective) (Directive) (Nondirective) Carbon-Carbon Multiple
Bonds
87 13
CH 2 NH 2
62 38
CH 2 N CH 3 2
CH 2 OH 95 5 48 52
CH=O 93 7 42 58
CN 75 25 20 80
CH=NOH 65 35 45 55
44 56
CH 2 OCH 3
33 67
CH 2 NHCOCH 3
CO 2 Na (or K) 55 45 30 70
CO 2 H 18 82 17 83
15 85 16 84
CO 2 CH 3
10 90 33 67
CONH 2
14 86 22 78
COCH 3
a. In methoxyethanol.
b. In ethanol.
The general ordering of aminomethyl > hydroxymethyl > CH=O > ester suggests
that Lewis basicity is the dominant factor in the directive effect. Problem 5.2 involves
considering the ordering of the various acyl substituents in more detail.
H C CH 3 CH 3 CH 3
2
X X
H , Pd/C
2
EtOH
5
Substituted indenes provide other examples of substituent directive effects. Over
Pd-alumina, the indenols 6a-c show both cis stereoselectivity and a syn directive effect.
The directive effect is reinforced by steric effects as the alkyl group becomes larger. 7
CH 3 H CH 3
H CH 3
O CH CH 3
CH 3 Pd-Al 2 3 3 + H
H H
OH 2 OH
R R OH R
6 trans,trans-7 cis,cis-7
R
CH 3 88 12
C H 97 3
2 5
(CH ) CH 100 0
3 2
Several indanes (8) were reduced to hexahydroindanes over Rh-Al O . The stereo-
3
2
chemistry of the ring junction is established at the stage of the reduction of the
tetrasubstituted double bonds. Only the amino group shows a strong directive effect. 8
7 K. Borszeky, T. Mallat, and A. Baiker, J. Catalysis, 188, 413 (1999).
8
V. S. Ranade, G. Consiglio, and R. Prins, J. Org. Chem., 65, 1132 (2000); V. S. Ranade, G. Consiglio,
and R. Prins, J. Org. Chem., 64, 8862 (1999).