Page 396 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 396
described as small-molecule reagents in solution. As understanding of the chemistry 369
of soluble hydrogenation catalysts developed, it became possible to extrapolate the
mechanistic concepts to heterogeneous catalysts. It is known that hydrogen is adsorbed SECTION 5.1
onto the metal surface, forming metal hydrogen bonds similar to those in transition Addition of Hydrogen at
Carbon-Carbon Multiple
metal hydrides. Alkenes are also adsorbed on the catalyst surface and at least three Bonds
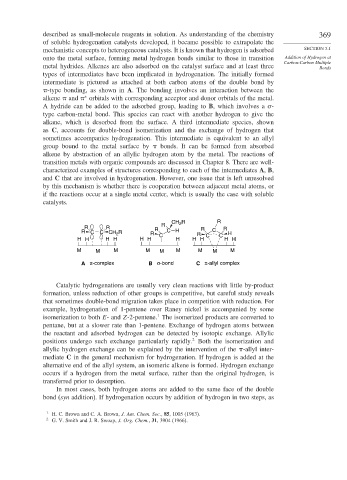
types of intermediates have been implicated in hydrogenation. The initially formed
intermediate is pictured as attached at both carbon atoms of the double bond by
-type bonding, as shown in A. The bonding involves an interaction between the
∗
alkene and orbitals with corresponding acceptor and donor orbitals of the metal.
A hydride can be added to the adsorbed group, leading to B, which involves a -
type carbon-metal bond. This species can react with another hydrogen to give the
alkane, which is desorbed from the surface. A third intermediate species, shown
as C, accounts for double-bond isomerization and the exchange of hydrogen that
sometimes accompanies hydrogenation. This intermediate is equivalent to an allyl
group bound to the metal surface by bonds. It can be formed from absorbed
alkene by abstraction of an allylic hydrogen atom by the metal. The reactions of
transition metals with organic compounds are discussed in Chapter 8. There are well-
characterized examples of structures corresponding to each of the intermediates A, B,
and C that are involved in hydrogenation. However, one issue that is left unresolved
by this mechanism is whether there is cooperation between adjacent metal atoms, or
if the reactions occur at a single metal center, which is usually the case with soluble
catalysts.
CH R R
R 2
R R R R
R C C CH 2 R R R C H R C H
H H H H H H C H H H C C H H
M M M M M M M M M
A π-complex B σ-bond C π-allyl complex
Catalytic hydrogenations are usually very clean reactions with little by-product
formation, unless reduction of other groups is competitive, but careful study reveals
that sometimes double-bond migration takes place in competition with reduction. For
example, hydrogenation of 1-pentene over Raney nickel is accompanied by some
1
isomerization to both E- and Z-2-pentene. The isomerized products are converted to
pentane, but at a slower rate than 1-pentene. Exchange of hydrogen atoms between
the reactant and adsorbed hydrogen can be detected by isotopic exchange. Allylic
2
positions undergo such exchange particularly rapidly. Both the isomerization and
allylic hydrogen exchange can be explained by the intervention of the -allyl inter-
mediate C in the general mechanism for hydrogenation. If hydrogen is added at the
alternative end of the allyl system, an isomeric alkene is formed. Hydrogen exchange
occurs if a hydrogen from the metal surface, rather than the original hydrogen, is
transferred prior to desorption.
In most cases, both hydrogen atoms are added to the same face of the double
bond (syn addition). If hydrogenation occurs by addition of hydrogen in two steps, as
1 H. C. Brown and C. A. Brown, J. Am. Chem. Soc., 85, 1005 (1963).
2
G. V. Smith and J. R. Swoap, J. Org. Chem., 31, 3904 (1966).