Page 404 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 404
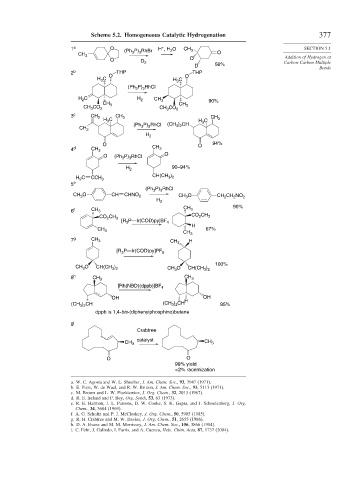
Scheme 5.2. Homogeneous Catalytic Hydrogenation 377
+
1 a O (Ph 3 P) 3 RhBr H , H 2 O CH 3 O SECTION 5.1
CH 3 Addition of Hydrogen at
O D
D 2 Carbon-Carbon Multiple
D 56%
Bonds
2 b O THP THP
H 3 C H 3 C O
(Ph 3 P) 3 RhCl
H 2 C H 2 CH 3 90%
CH 3 CH 3
CH 3 CO 2 CH 3 CO 2
3 c CH 2 CH 3
H 3 C H 3 C CH 3
(Ph 3 P) 3 RhCl (CH 3 ) 2 CH
CH 3
H 2
O O 94%
4 d CH 3 CH 3
O (Ph 3 P) 3 RhCl O
90–94%
H 2
H 2 C CCH 3 CH(CH 3 ) 2
5 e
(Ph 3 P) 3 RhCl
CH 3 O CH CHNO 2 CH 3 O CH 2 CH 2 NO 2
H 2
90%
6 f CH 3 CH 3
CO 2 CH 3
CO 2 CH 3
[R 3 P—Ir(COD)py]BF 4
H
CH 3 67%
CH 3
7 g CH 3 H
CH 3
[R 3 P—Ir(COD)py]PF 6
100%
CH 3 O CH(CH 3 ) 2 CH 3 O CH(CH 3 ) 2
8 h CH 3 CH 3
[Rh(NBD)(dppb)]BF 4
OH OH
(CH 3 ) 2 CH (CH 3 ) 2 CH H 95%
dppb is 1,4-bis-(diphenylphosphino)butane
9 i
Crabtree
catalyst
CH 3 CH 3
O O
99% yield
< 2% racemization
a. W. C. Agosta and W. L. Shreiber, J. Am. Chem. Soc., 93, 3947 (1971).
b. E. Piers, W. de Waal, and R. W. Britton, J. Am. Chem. Soc., 93, 5113 (1971).
c. M. Brown and L. W. Piszkiewicz, J. Org. Chem., 32, 2013 (1967).
d. R. E. Ireland and P. Bey, Org. Synth, 53, 63 (1973).
e. R. E. Harmon, J. L. Parsons, D. W. Cooke, S. K. Gupta, and J. Schoolenberg, J. Org.
Chem., 34, 3684 (1969).
f. A. G. Schultz and P. J. McCloskey, J. Org. Chem., 50, 5905 (1985).
g. R. H. Crabtree and M. W. Davies, J. Org. Chem., 51, 2655 (1986).
h. D. A. Evans and M. M. Morrissey, J. Am. Chem. Soc., 106, 3866 (1984).
i. C. Fehr, J. Galindo, I. Farris, and A. Cuenca, Helv. Chim. Acta, 87, 1737 (2004).