Page 409 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 409
382
CHAPTER 5
Reduction of
Carbon-Carbon Multiple
Bonds, Carbonyl
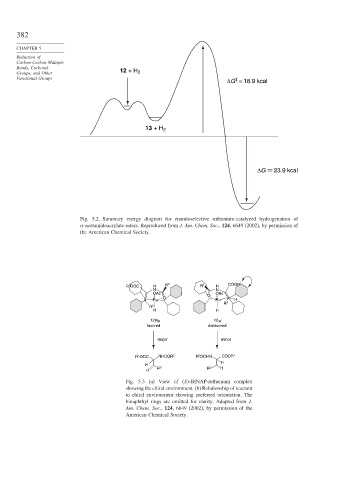
Groups, and Other 12 + H 2
Functional Groups ‡
ΔG = 18.9 kcal
13 + H 2
ΔG 23.9 kcal
Fig. 5.2. Summary energy diagram for enantioselective ruthenium-catalyzed hydrogenation of
-acetamidoacrylate esters. Reproduced from J. Am. Chem. Soc., 124, 6649 (2002), by permission of
the American Chemical Society.
1
R OOC H R 2 R 2 H COOR 1
N N
OAc O OAc
Ru O Ru H
H R 3
R 3
H H
12 Re 12 si
favored disfavored
major minor
1
2
R OOC NHCOR 2 R OCHN COOR 1
H
H
R 3 R 3 H
H
Fig. 5.3. (a) View of (S)-BINAP-ruthenium complex
showing the chiral environment. (b) Relationship of reactant
to chiral environment showing preferred orientation. The
binaphthyl rings are omitted for clarity. Adapted from J.
Am. Chem. Soc., 124, 6649 (2002), by permission of the
American Chemical Society.