Page 411 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 411
384
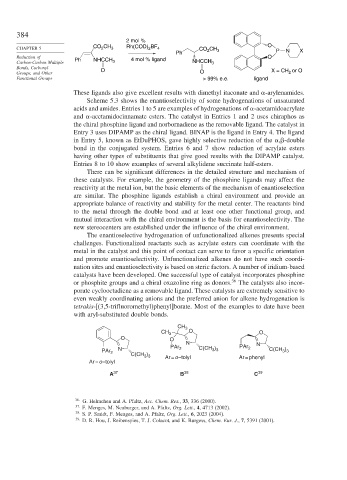
2 mol %
CO CH BF O
CHAPTER 5 2 3 Rh(COD) 2 4 CO 2 CH
Ph 3 P N X
Reduction of Ph NHCCH 4 mol % ligand O
Carbon-Carbon Multiple 3 NHCCH 3
Bonds, Carbonyl O
Groups, and Other O X = CH 2 or O
Functional Groups > 99% e.e. ligand
These ligands also give excellent results with dimethyl itaconate and -arylenamides.
Scheme 5.3 shows the enantioselectivity of some hydrogenations of unsaturated
acids and amides. Entries 1 to 5 are examples of hydrogenations of -acetamidoacrylate
and -acetamidocinnamate esters. The catalyst in Entries 1 and 2 uses chiraphos as
the chiral phosphine ligand and norbornadiene as the removable ligand. The catalyst in
Entry 3 uses DIPAMP as the chiral ligand. BINAP is the ligand in Entry 4. The ligand
in Entry 5, known as EtDuPHOS, gave highly selective reduction of the , -double
bond in the conjugated system. Entries 6 and 7 show reduction of acrylate esters
having other types of substituents that give good results with the DIPAMP catalyst.
Entries 8 to 10 show examples of several alkylidene succinate half-esters.
There can be significant differences in the detailed structure and mechanism of
these catalysts. For example, the geometry of the phosphine ligands may affect the
reactivity at the metal ion, but the basic elements of the mechanism of enantioselection
are similar. The phosphine ligands establish a chiral environment and provide an
appropriate balance of reactivity and stability for the metal center. The reactants bind
to the metal through the double bond and at least one other functional group, and
mutual interaction with the chiral environment is the basis for enantioselectivity. The
new stereocenters are established under the influence of the chiral environment.
The enantioselective hydrogenation of unfunctionalized alkenes presents special
challenges. Functionalized reactants such as acrylate esters can coordinate with the
metal in the catalyst and this point of contact can serve to favor a specific orientation
and promote enantioselectivity. Unfunctionalized alkenes do not have such coordi-
nation sites and enantioselectivity is based on steric factors. A number of iridium-based
catalysts have been developed. One successful type of catalyst incorporates phosphine
or phosphite groups and a chiral oxazoline ring as donors. 36 The catalysts also incor-
porate cyclooctadiene as a removable ligand. These catalysts are extremely sensitive to
even weakly coordinating anions and the preferred anion for alkene hydrogenation is
tetrakis-[(3,5-trifluoromethyl)phenyl]borate. Most of the examples to date have been
with aryl-substituted double bonds.
CH 3
CH 3 O O
O O
N PAr N
3 3
PAr 2 N C(CH ) PAr 2 C(CH ) 2 C(CH )
3 3
Ar = o –tolyl 3 3 Ar = o –tolyl Ar = phenyl
A 37 B 38 C 39
36
G. Helmchen and A. Pfaltz, Acc. Chem. Res., 33, 336 (2000).
37
F. Menges, M. Neuburger, and A. Pfaltz, Org. Lett., 4, 4713 (2002).
38 S. P. Smidt, F. Menges, and A. Pfaltz, Org. Lett., 6, 2023 (2004).
39
D. R. Hou, J. Reibenspies, T. J. Colacot, and K. Burgess, Chem. Eur. J., 7, 5391 (2001).