Page 410 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 410
H 383
+
P
H H SECTION 5.1
Ru(MeCN) n (THF) 3-n
CO CH COO CH
2 3 P 2 3 Addition of Hydrogen at
Ph Ph
n = 0–2 Carbon-Carbon Multiple
H NHCOCH NHCOCH 3 Bonds
3
(R)-MACH MAC
2
MAC
H
2
CH
3
+ +
O H H
N Ph
P O P O CH 3
Ru O Ru H
P CH P N
3
N Ph N O H
H H O
C C
CH 3
CH CH 3
3
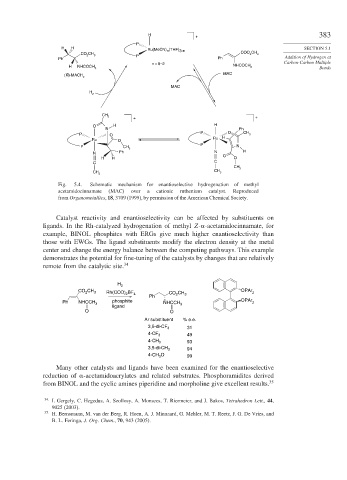
Fig. 5.4. Schematic mechanism for enantioselective hydrogenation of methyl
acetamidocinnamate (MAC) over a cationic ruthenium catalyst. Reproduced
from Organometallics, 18, 3709 (1999), by permission of the American Chemical Society.
Catalyst reactivity and enantioselectivity can be affected by substituents on
ligands. In the Rh-catalyzed hydrogenation of methyl Z- -acetamidocinnamate, for
example, BINOL phosphites with ERGs give much higher enantioselectivity than
those with EWGs. The ligand substituents modify the electron density at the metal
center and change the energy balance between the competing pathways. This example
demonstrates the potential for fine-tuning of the catalysts by changes that are relatively
remote from the catalytic site. 34
H 2
OPAr
CO 2 CH 3
Rh(COD) 2 BF 4 CO 2 CH 3 2
Ph
Ph NHCCH 3 phosphite NHCCH 3 OPAr 2
ligand
O O
Ar substituent % e.e.
3,5-di-CF 3 31
49
4-CF 3
4-CH 3 93
3,5-di-CH 3 94
4-CH 3 O 99
Many other catalysts and ligands have been examined for the enantioselective
reduction of -acetamidoacrylates and related substrates. Phosphoramidites derived
from BINOL and the cyclic amines piperidine and morpholine give excellent results. 35
34 I. Gergely, C. Hegedus, A. Szollosy, A. Monsees, T. Riermeier, and J. Bakos, Tetrahedron Lett., 44,
9025 (2003).
35
H. Bernsmann, M. van der Berg, R. Hoen, A. J. Minnaard, G. Mehler, M. T. Reetz, J. G. De Vries, and
B. L. Feringa, J. Org. Chem., 70, 943 (2005).