Page 413 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 413
386 Scheme 5.3. (Continued)
CHAPTER 5 Reactant Catalyst Product Configuration % e.e.
Reduction of 9 g
Carbon-Carbon Multiple Ph PPh 2 S >95
Bonds, Carbonyl HC PhCH 2
Groups, and Other CH 3 O 2 C CO 2 H Rh CO 2 H
Functional Groups N HO 2 C
Ph 2 PCH 2 CNHPh
O
10 h
CH 2 (C 6 H 11 ) 2 S 96
CO 2 H CH 3
CH 3 O 2 C P CO 2 H
Rh CH 3 HO 2 C
P CH 3
(C 6 H 11 ) 2
a. M. D. Fryzuk and B. Bosnich, J. Am. Chem. Soc. 99, 6262 (1977).
b. B. D. Vineyard, W. S. Knowles, M. J. Sabacky, G. L. Bachman, and D. J. Weinkauff, J. Am.
Chem. Soc., 99, 5946 (1977).
c. A. Miyashita, H. Takaya, T. Souchi, and R. Noyori, Tetrahedron, 40, 1245 (1984).
d. M. J. Burk, J. G. Allen, and W. F. Kiesman, J. Am. Chem. Soc., 120, 657 (1998).
e. W. C. Christopfel and B. D. Vineyard, J. Am. Chem. Soc., 101, 4406 (1979).
f. M. J. Burk, F. Bienewald, M. Harris, and A. Zanotti-Gerosa, Angew. Chem. Int. Ed. Engl. 37,
1931 (1998).
g. H. Jendralla, Tetrahedron Lett., 32, 3671 (1991).
h. T. Chiba, A. Miyashita, H. Nohira, and H. Takaya, Tetrahedron Lett., 32, 4745 (1991).
CH 3 CH 3 CH 3
CH 3
CH 3
CH O
CH O 3
3
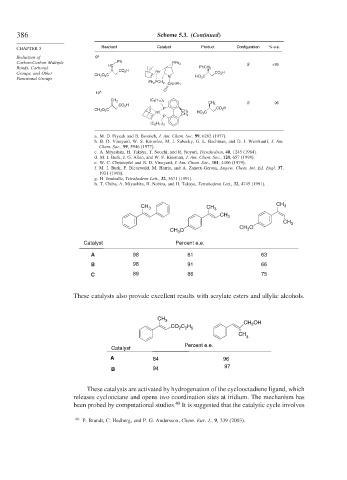
Catalyst Percent e.e.
A 98 81 63
B 98 91 66
C 89 86 75
These catalysts also provide excellent results with acrylate esters and allylic alcohols.
CH 3 CH OH
CO C H 2
2 2 5
CH
3
Percent e.e.
Catalyst
A 84 96
97
B 94
These catalysts are activated by hydrogenation of the cyclooctadiene ligand, which
releases cyclooctane and opens two coordination sites at iridium. The mechanism has
40
been probed by computational studies. It is suggested that the catalytic cycle involves
40
P. Brandt, C. Hedberg, and P. G. Andersson, Chem. Eur. J., 9, 339 (2003).