Page 417 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 417
390 Scheme 5.4. (Continued)
CHAPTER 5 j
10 S S
CH CH 3
Reduction of CH 3 3 CH 3
Carbon-Carbon Multiple N O O KO 2 CN NCO 2 K N O O
Bonds, Carbonyl OH OH
Groups, and Other CH 3 CO 2 H
CH 3 CH 3
Functional Groups CH 3 CH 3 CH 3 CH 3
CH 3
CH 3
O O
OH OH
CH 3 CH 3 86%
a. E. E. van Tamelen, R. S. Dewey, and R. J. Timmons, J. Am. Chem. Soc., 83, 3725 (1961).
b. E. E. van Tamelen, R. S. Dewey, M. F. Lease, and W. H. Pirkle, J. Am. Chem. Soc., 83, 4302 (1961).
c. M Ohno, and M. Okamoto, Org. Synth., 49, 30 (1969).
d. W. Durckheimer, Liebigs Ann. Chem., 712, 240 (1969).
e. L. A. Paquette, A. R. Browne, E. Chamot, and J. F. Blount, J. Am. Chem. Soc., 102, 643 (1980).
f. J.-M. Durgnat and P. Vogel, Helv. Chim. Acta, 76, 222 (1993).
g. P. A. Grieco, R. Lis, R. E. Zelle, and J. Finn, J. Am. Chem. Soc., 108, 5908 (1986).
h. P. Magnus, T. Gallagher, P. Brown, and J. C. Huffman, J. Am. Chem. Soc., 106, 2105 (1984).
i. M. Squillacote, J. DeFelippis, and Y. L. Lai, Tetrahedron Lett., 34, 4137 (1993).
j. K. Biswas, H. Lin, J. T. Njgardson, M. D. Chappell, T.-C. Chou, Y. Guan, W. P. Tong, L. He, S. B. Horwitz,
and S. J. Danishefsky, J. Am. Chem. Soc., 124, 9825 (2002).
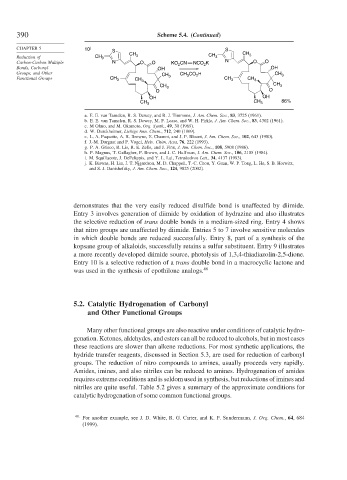
demonstrates that the very easily reduced disulfide bond is unaffected by diimide.
Entry 3 involves generation of diimide by oxidation of hydrazine and also illustrates
the selective reduction of trans double bonds in a medium-sized ring. Entry 4 shows
that nitro groups are unaffected by diimide. Entries 5 to 7 involve sensitive molecules
in which double bonds are reduced successfully. Entry 8, part of a synthesis of the
kopsane group of alkaloids, successfully retains a sulfur substituent. Entry 9 illustrates
a more recently developed diimide source, photolysis of 1,3,4-thiadiazolin-2,5-dione.
Entry 10 is a selective reduction of a trans double bond in a macrocyclic lactone and
was used in the synthesis of epothilone analogs. 48
5.2. Catalytic Hydrogenation of Carbonyl
and Other Functional Groups
Many other functional groups are also reactive under conditions of catalytic hydro-
genation. Ketones, aldehydes, and esters can all be reduced to alcohols, but in most cases
these reactions are slower than alkene reductions. For most synthetic applications, the
hydride transfer reagents, discussed in Section 5.3, are used for reduction of carbonyl
groups. The reduction of nitro compounds to amines, usually proceeds very rapidly.
Amides, imines, and also nitriles can be reduced to amines. Hydrogenation of amides
requires extreme conditions and is seldom used in synthesis, but reductions of imines and
nitriles are quite useful. Table 5.2 gives a summary of the approximate conditions for
catalytic hydrogenation of some common functional groups.
48
For another example, see J. D. White, R. G. Carter, and K. F. Sundermann, J. Org. Chem., 64, 684
(1999).