Page 422 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 422
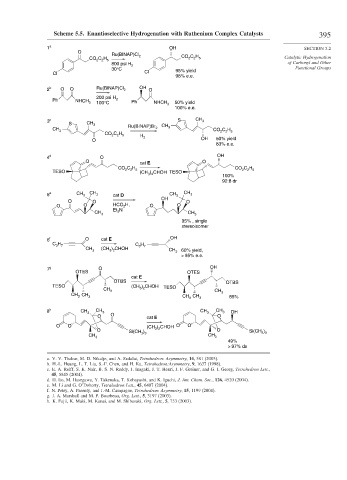
Scheme 5.5. Enantioselective Hydrogenation with Ruthenium Complex Catalysts 395
1 a OH SECTION 5.2
O
Ru(BINAP)Cl 2 CO C H
C H 2 2 5 Catalytic Hydrogenation
CO 2 2 5
800 psi H 2 of Carbonyl and Other
30°C 95% yield Functional Groups
Cl Cl
98% e.e.
2 b O O Ru(BINAP)Cl 2 OH O
200 psi H
Ph NHCH 3 100°C 2 Ph NHCH 50% yield
3
100% e.e.
3 c S CH 3
S CH 3
CH 3 Ru(BINAP)Br 2 CH 3 CO C H 5
2
2
CO 2 C H
2 5 H 2
O OH 50% yield
83% e.e.
4 d O OH
O O
cat E
C H CO C H
CO 2 2 5
TESO (CH ) CHOH TESO 2 2 5
3 2
100%
92:8 dr
5 e CH 3 CH 3 cat D CH 3 CH 3
OH
O O O
O O HCO 2 H, O O
N
CH 3 Et 3 CH 3
95% , single
stereoisomer
6 f O cat E OH
C H 7 C H
3
CH (CH ) CHOH 3 7 CH
3 3 2 3 60% yield,
> 95% e.e.
7 g O OH
OTES OTES
cat E
OTBS OTBS
TESO (CH ) CHOH TESO
CH 3 3 2 CH
CH 3 CH 3 CH CH 3 3 85%
3
8 h CH 3 CH 3 CH 3 CH 3 OH
O O cat E O
O O (CH ) CHOH O O
O ) 3 2 O Si(CH )
CH Si(CH 3 3 CH 3 3
3 3
49%
> 97% ds
a. V. V. Thakur, M. D. Nikalje, and A. Sudalai, Tetrahedron: Asymmetry, 14, 581 (2003).
b. H.-L. Huang, L. T. Liu, S.-F. Chen, and H. Ku, Tetrahedron:Asymmetry, 9, 1637 (1998).
c. E. A. Reiff, S. K. Nair, B. S. N. Reddy, J. Inagaki, J. T. Henri, J. F. Greiner, and G. I. Georg, Tetrahedron Lett.,
45, 5845 (2004).
d. H. Ito, M. Hasegawa, Y. Takenaka, T. Kobayashi, and K. Iguchi, J. Am. Chem. Soc., 126, 4520 (2004).
e. M. Li and G. O’Doherty, Tetrahedron Lett., 45, 6407 (2004).
f. N. Petry, A. Parenty, and J.-M. Campagne, Tetrahedron: Asymmetry, 15, 1199 (2004).
g. J. A. Marshall and M. P. Bourbeau, Org. Lett., 5, 3197 (2003).
h. K. Fujii, K. Maki, M. Kanai, and M. Shibasaki, Org. Lett., 5, 733 (2003).