Page 420 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 420
H H 393
H H 2
P N 2 H P N SECTION 5.2
Ru 2 Ru
Catalytic Hydrogenation
N N of Carbonyl and Other
P H P H
H 2 H 2 – H Functional Groups
+
H +
H Ru NH H
P N 2 P H N 2
Ru δ – H H δ + Ru
N N
P H C O P H
H H 2
CHOH
R 2 R C O
2
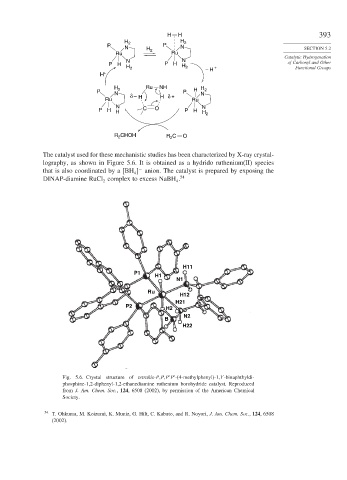
The catalyst used for these mechanistic studies has been characterized by X-ray crystal-
lography, as shown in Figure 5.6. It is obtained as a hydrido ruthenium(II) species
that is also coordinated by a
BH anion. The catalyst is prepared by exposing the
−
4
DINAP-diamine RuCl complex to excess NaBH . 54
2 4
H11
P1
H1
N1
Ru
H12
H21
P2 H2
N2
B
H22
Fig. 5.6. Crystal structure of tetrakis-P,P,P P -(4-methylphenyl)-1,1 -binaphthyldi-
phosphine-1,2-diphenyl-1,2-ethanediamine ruthenium borohydride catalyst. Reproduced
from J. Am. Chem. Soc., 124, 6508 (2002), by permission of the American Chemical
Society.
54
T. Ohkuma, M. Koizumi, K. Muniz, G. Hilt, C. Kabuto, and R. Noyori, J. Am. Chem. Soc., 124, 6508
(2002).