Page 408 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 408
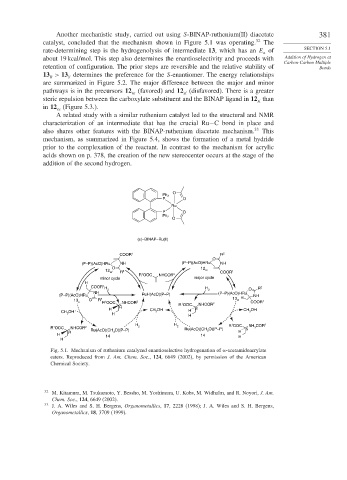
Another mechanistic study, carried out using S-BINAP-ruthenium(II) diacetate 381
catalyst, concluded that the mechanism shown in Figure 5.1 was operating. 32 The
rate-determining step is the hydrogenolysis of intermediate 13, which has an E of SECTION 5.1
a
about 19 kcal/mol. This step also determines the enantioselectivity and proceeds with Addition of Hydrogen at
Carbon-Carbon Multiple
retention of configuration. The prior steps are reversible and the relative stability of Bonds
13 > 13 determines the preference for the S-enantiomer. The energy relationships
R
S
are summarized in Figure 5.2. The major difference between the major and minor
pathways is in the precursors 12 (favored) and 12 (disfavored). There is a greater
re si
steric repulsion between the carboxylate substituent and the BINAP ligand in 12 than
si
in 12 (Figure 5.3.).
re
A related study with a similar ruthenium catalyst led to the structural and NMR
characterization of an intermediate that has the crucial Ru−C bond in place and
also shares other features with the BINAP-ruthenium diacetate mechanism. 33 This
mechanism, as summarized in Figure 5.4, shows the formation of a metal hydride
prior to the complexation of the reactant. In contrast to the mechanism for acrylic
acids shown on p. 378, the creation of the new stereocenter occurs at the stage of the
addition of the second hydrogen.
O
Ph 2
P O
Ru
P P O
Ph 2
O
(s)–BINAP–Ru(ll)
COOR 1 R 2
O
(P–P)(AcO)HRu NH (P–P)(AcO)HRu NH
O 12
12 si R 2 re COOR 1
1
R OOC NHCOR 2
minor cycle major cycle
H
1
COOR H H O R 2
2 2
NH (P–P)(AcO)HRu
(P–P)(AcO)HRu RuH(AcO)(P–P) NH
13 O R 2 13 H
R
s R OOC NHCOR 2 1 NHCOR 2 COOR 1
1
R R OOC
H CH OH S OH
OH H CH 3
H H
CH 3 3
H H R OOC NH COR 2
1
1
R OOC NHCOR 2 O)(P–P) 2 2 Ru(AcO)(CH O)(P–P) S 3
R Ru(AcO)(CH 3 3 H
H 14 14
H H
Fig. 5.1. Mechanism of ruthenium catalyzed enantioselective hydrogenation of -acetamidoacrylate
esters. Reproduced from J. Am. Chem. Soc., 124, 6649 (2002), by permission of the American
Chemical Society.
32 M. Kitamura, M. Tsukamoto, Y. Bessho, M. Yoshimura, U. Kobs, M. Widhalm, and R. Noyori, J. Am.
Chem. Soc., 124, 6649 (2002).
33
J. A. Wiles and S. H. Bergens, Organometallics, 17, 2228 (1998); J. A. Wiles and S. H. Bergens,
Organometallics, 18, 3709 (1999).