Page 406 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 406
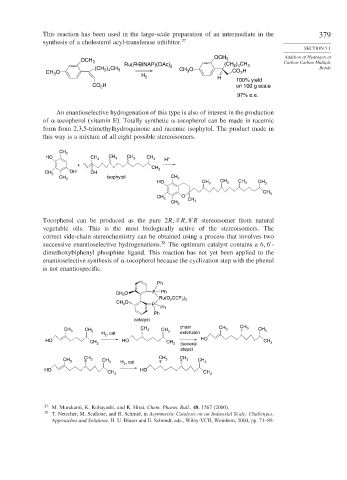
This reaction has been used in the large-scale preparation of an intermediate in the 379
synthesis of a cholesterol acyl-transferase inhibitor. 27
SECTION 5.1
OCH Addition of Hydrogen at
OCH 3 3 Carbon-Carbon Multiple
) CH
Ru(R-BINAP)(OAc) 2 (CH 2 4 3
(CH ) CH CH O Bonds
CH O 2 4 3 H 3 CO H
2
3
H
2
100% yield
H
CO 2 on 100 g scale
97% e.e.
An enantioselective hydrogenation of this type is also of interest in the production
of -tocopherol (vitamin E). Totally synthetic -tocopherol can be made in racemic
form from 2,3,5-trimethylhydroquinone and racemic isophytol. The product made in
this way is a mixture of all eight possible stereoisomers.
CH 3
HO CH 3 CH 3 CH 3 CH 3 H +
+
CH
CH 3 OH OH 3
CH isophytol CH 3
3 CH
HO CH 3 3 CH 3 CH 3
CH
CH O 3
3 CH
CH 3
3
Tocopherol can be produced as the pure 2R 4 R 8 R stereoisomer from natural
vegetable oils. This is the most biologically active of the stereoisomers. The
correct side-chain stereochemistry can be obtained using a process that involves two
successive enantioselective hydrogenations. 28 The optimum catalyst contains a 6 6 -
dimethoxybiphenyl phosphine ligand. This reaction has not yet been applied to the
enantioselective synthesis of -tocopherol because the cyclization step with the phenol
is not enantiospecific.
Ph
CH O P Ph
3
Ru(O CCF )
O 2 3 2
CH 3
P
Ph
Ph
catalyst
CH CH CH 3 CH chain CH 3 CH 3 CH
3 3 3 extension 3
H , cat
2
HO
HO CH 3 HO CH 3 (several CH 3
steps)
CH CH 3 CH CH 3 CH 3 CH
3
3
H 2 , cat 3
HO HO
CH CH
3 3
27 M. Murakami, K. Kobayashi, and K. Hirai, Chem. Pharm. Bull., 48, 1567 (2000).
28
T. Netscher, M. Scalione, and R. Schmid, in Asymmetric Catalysis on an Industrial Scale: Challenges,
Approaches and Solutions, H. U. Blaser and E. Schmidt, eds., Wiley-VCH, Weinhem, 2004, pp. 71–89.