Page 387 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 387
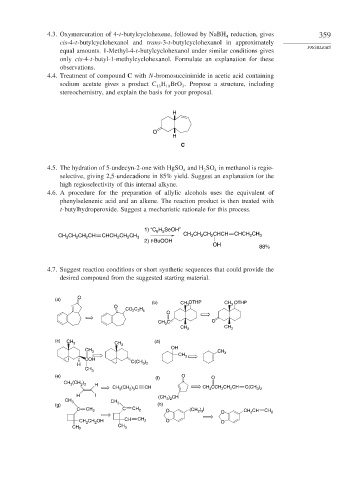
4.3. Oxymercuration of 4-t-butylcyclohexene, followed by NaBH reduction, gives 359
4
cis-4-t-butylcyclohexanol and trans-3-t-butylcyclohexanol in approximately
PROBLEMS
equal amounts. 1-Methyl-4-t-butylcyclohexanol under similar conditions gives
only cis-4-t-butyl-1-methylcyclohexanol. Formulate an explanation for these
observations.
4.4. Treatment of compound C with N-bromosuccinimide in acetic acid containing
sodium acetate gives a product C H BrO . Propose a structure, including
13
3
19
stereochemistry, and explain the basis for your proposal.
H
O
H
C
4.5. The hydration of 5-undecyn-2-one with HgSO and H SO in methanol is regio-
4
4
2
selective, giving 2,5-undecadione in 85% yield. Suggest an explanation for the
high regioselectivity of this internal alkyne.
4.6. A procedure for the preparation of allylic alcohols uses the equivalent of
phenylselenenic acid and an alkene. The reaction product is then treated with
t-butylhydroperoxide. Suggest a mechanistic rationale for this process.
1) “C H SeOH”
6 5
CH CH CH CH CHCH CH CH 3 CH CH CH CHCH CHCH 2 CH 3
2
2
3
2
2
3
2
2
2) t-BuOOH
OH 88%
4.7. Suggest reaction conditions or short synthetic sequences that could provide the
desired compound from the suggested starting material.
(a) O
(b) CH OTHP CH OTHP
O 3 3
2
CO 2 C H 5
O
CH C O
3
CH 3 CH 3
(c) CH (d )
3 CH 3
CH 3 OH CH
CH 3 3
COH
3 2
H C(CH )
CH 3
(e) O
(f) O
CH (CH ) H
3
2 3
CH (CH ) C CH CH CCH CH CH C(CH )
3 2 3 3 2 2 3 2
H I (CH ) CH
CH 3 CH 3 2
(g) 3 (h)
C CH 2 C CH 2 O (CH ) I O CH CH CH 2
2 3
2
CH CH OH CH CH 2 O O
2 2
CH
CH 3 3