Page 491 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 491
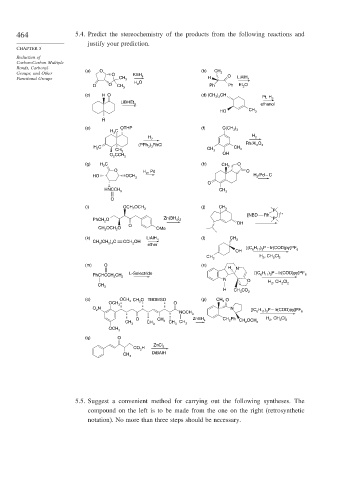
464 5.4. Predict the stereochemistry of the products from the following reactions and
justify your prediction.
CHAPTER 5
Reduction of
Carbon-Carbon Multiple
Bonds, Carbonyl
Groups, and Other (a) O O KBH (b) CH 3 O
Functional Groups CH 3 4 H LiAlH 4
2
O O CH 3 H O Ph Ph Et O
2
(c) H O (d) (CH ) CH Pt, H
3 2
LiBHEt 2
3 ethanol
HO CH 3
H
(e) OTHP (f) C(CH )
H 3 C 3 3
H H 2
2
Rh/Al O
(PPh ) RhCl 2 3
C 3 3
H 2 CH CH 3
CH 3 3
O CCH OH
2 3
(g) C (h) O
H 2
CH 3
O H , Pd O
HO OCH 3 2 H /Pd – C
2
O
HNCCH CH
3 3
O
(i) OCH OCH (j) CH
2 3 3
P
[NBD Rh ] 1+
PhCH O Zn(BH ) 4 2 P
2 OH
O
CH OCH O OMe
3 2
(k) LiAlH (l) CH
CH (CH ) C CCH 2 OH 4 3
3 2 4
ether
[(C H ) P – Ir(COD)py]PF
OH 6 11 3 6
CH H , CH Cl
3 2 2 2
(m) O (n)
H N
11 3
6
PhCHCCH CH 3 L-Selectride [(C H ) P – Ir(COD)py]PF 6
2
N O H , CH Cl
CH 3 2 2 2
H CO
CH 3
2
(o) OCH CH 3 O TBDMSO (p) CH 3 O
OCH 3 O
3
O N N
2 [(C H ) P – Ir(COD)py]PF
NOCH 6 11 3 6
3
O CH ZnBH CH Ph H 2 , CH 2 Cl 2
CH CH 3 3 CH CH 4 2 CH OCH 3
2
3 3 3
OCH
3
(q) O
ZnCl
H 2
CO 2
CH 3 DiBAlH
5.5. Suggest a convenient method for carrying out the following syntheses. The
compound on the left is to be made from the one on the right (retrosynthetic
notation). No more than three steps should be necessary.