Page 496 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 496
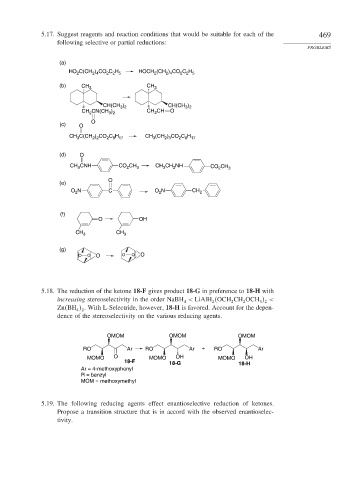
5.17. Suggest reagents and reaction conditions that would be suitable for each of the 469
following selective or partial reductions:
PROBLEMS
(a)
HO C(CH ) CO C H HOCH (CH ) CO C H
2
2
2 2 5
2 2 5
2 4
2 4
(b) CH 3 CH 3
CH(CH ) CH(CH )
3 2
3 2
CH CN(CH ) CH CH O
3 2
2
2
O
(c) O
CH C(CH ) CO C H CH 3 (CH ) CO C H
2 8 17
2 8 17
2 2
2 3
3
(d) O
CH CNH CO CH 3 CH CH NH CO CH 3
2
3
2
3
2
O
(e)
O N C O N CH 2
2
2
(f)
O OH
CH 3 CH 3
(g)
O O O O O O
5.18. The reduction of the ketone 18-F gives product 18-G in preference to 18-H with
increasing stereoselectivity in the order NaBH < LiAlH OCH CH OCH <
2
3 2
2
4
2
Zn BH . With L-Selectride, however, 18-H is favored. Account for the depen-
4 2
dence of the stereoselectivity on the various reducing agents.
OMOM OMOM OMOM
RO Ar RO Ar + RO Ar
MOMO O MOMO OH MOMO OH
18-F 18-G 18-H
Ar = 4-methoxyphenyl
R = benzyl
MOM = methoxymethyl
5.19. The following reducing agents effect enantioselective reduction of ketones.
Propose a transition structure that is in accord with the observed enantioselec-
tivity.