Page 498 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 498
(a) PhCH O OCH Ph (b) (CH ) CH CHCO CH 471
2
2
O CH SmI 2 2 3 2 3 SmI 2 PROBLEMS
CH O
CH O
PhCH O OCH 2 Ph 3
2
(c) CH 3 H (d) TBDMSO
O OH SmI 2 CH 3 O CO 2 CH 3 SmI 2
HMPA CH 3 O CH 3
O CH O
CH CCH 3
2
O
(f) Ph
(e) OTBDMS CO 2
O CH CO CH 3 SmI 2 (CH 3 3 CCH N CH O SmI 2
) SiC
2
2
O CH O CH OTBDMS
2
CH 3 O
CH 3
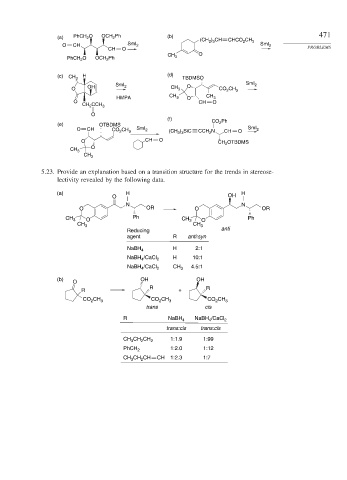
5.23. Provide an explanation based on a transition structure for the trends in stereose-
lectivity revealed by the following data.
(a) H H
O OH
N N
O OR O OR
CH 3 O Ph CH 3 O Ph
CH 3 CH 3
Reducing anti
agent R anti:syn
H 2:1
NaBH 4
NaBH /CaCl 2 H 10:1
4
NaBH /CaCl 2 CH 3 4.5:1
4
(b) OH OH
O
R R
R +
CO CH 3 CO CH 3 CO CH 3
2
2
2
trans cis
R NaBH 4 NaBH /CaCl 2
4
trans:cis trans:cis
CH CH CH 2 1:1.9 1:99
2
3
PhCH 2 1:2.0 1:12
CH CH CH CH 1:2.3 1:7
2
3